-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2012; 2(6): 176-178
doi: 10.5923/j.materials.20120206.03
Thermodynamic Research of Solubility of Irradiate Cross-linked Copolymers in Mix Butadiene Nitrile with Polyvinylchloride
S. M. Mammadov1, S. A. Rzayeva1, A. A. Garibov1, E. O. Akperov2, J. S. Mammadov1
1Institute of Radiation Problems of ANAS, Baku, AZ1143, Azerbaijan
2Baku State University, Baku, AZ 1143, Azerbaijan
Correspondence to: S. M. Mammadov, Institute of Radiation Problems of ANAS, Baku, AZ1143, Azerbaijan.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
As shown, Energy effect have definition role for solubility of irradiate copolymer in mix butadiene nitrile rubber with polyvinylchloride, entropic effect remaining negative in initial polymer do not promote to solubility process. The experiments showed that increasing of radiation dosage during cross linking reduces characteristic viscosity of solutions and influence to change other physical-chemical properties of cross- linked copolymers.
Keywords: Solubility, Radiation, Copolymer,Butadiene Nitrile Rubber, Polyvinyl Chloride, Enthalpy, Characteristic Viscosity
Cite this paper: S. M. Mammadov, S. A. Rzayeva, A. A. Garibov, E. O. Akperov, J. S. Mammadov, "Thermodynamic Research of Solubility of Irradiate Cross-linked Copolymers in Mix Butadiene Nitrile with Polyvinylchloride", American Journal of Materials Science, Vol. 2 No. 6, 2012, pp. 176-178. doi: 10.5923/j.materials.20120206.03.
1. Introduction
- Despite the progress in study of physical-chemical properties of cross-linked copolymers which synthesized by radiation methods, such as the property of their solubility are not sufficiently explored. It’s known that, secondary processes (cross-linking or destruction), occurring in polymer components by influence ionize radiation, depends on dosage and power of radiation of the same system copolymers can be form products with solubility, restricted solubility or non solubility properties even in solvents which are common solvents for all polymers[1-9. Previously we and others[10, 11 have shown that, cross-linking polymers (elastopolymers) are characterized by very limited solubility in comparison with raw materials as result the influence of one compound to another. In this connection, studies of solubility of cross-linking copolymers are of interest which have the same solubility in raw materials and have the same solvent. This work describe the results of thermodynamical research irradiated cross-linked copolymers in mix butadiene nitrile rubber (BNR, SKN-40) with polyvinylchloride (PVC) depended on radiation dosage.
2. Experimental Part
- Copolymerization of BNR with PVC was carried out by mixing rubber latex with PVC (80:20). The mixture was filled with antirad (eg. 2-amino -4.6-bis-trichlomethylsym triasine) to reduce irradiation dosage because 2-amino-4.6-bis-trichlomethylsym triasine radiation sensitive monomer and coagulated with aluminum salts at 313-323K. the formed grit was washed with water and dried by 343K. Irradiation occurred by γ-rays Co60 in dosage interval 10-50kGy dose power 200r/s, by irradiation temperature 313K. The methods of experiments and the results of synthesis had been previously shown by us[12].
3. Results and Discussion
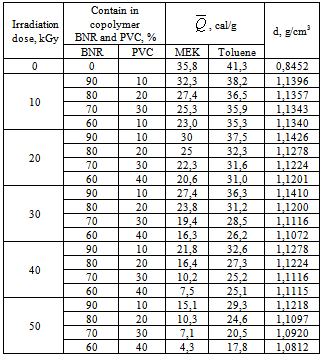
- Thermal effect of irradiated copolymers defined by 298K in microcalorimeter described in[13]. The scaling factor of calorimeter was equal to 0.0637cal/mm. Solution heat was defined for initial polymer BNR (70%) and second copolymer containing PVC 30%. The graphic method crossing clippings were used for defining the change of partial enthalpy of solvent (toluene) in system BNR-toluene and copolymer-toluene[14].Isotherms of sorption of toluene, BNR and copolymer were determined by the installation of sorption. For defining dependence of changing partial enthalpy toluene
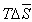 from solution concentration was used value of
from solution concentration was used value of 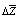 counted by sorption isotherms according to the known equation. The value of
counted by sorption isotherms according to the known equation. The value of 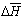 was found by graphic chart.The density of copolymers was determined by hydrostatic weighting method[15], based on the definition of the mass loss of sample immersed in the liquid, which is inert in relation to copolymers.The solubility of irradiated copolymers SKN-40PVC30 was qualitatively studied. The different solvents were tested. It was found that irradiated copolymers dissolve in solvents such as toluene, dichloroethane, solvent, methyl ethyl ketene, dioxane, chloroform. These dates show that obtained copolymer didn’t loose its ability to dissolution in the result of reaction, because their initial components nearby solubility and have the same solvents. But, as known, strictly scientific criteria dissolving ability of solvent is a change of Gibbs free enthalpy of solvent in system polymer-solvent include both sides of dissolve processes: enthalpy and entropy changes. Table 1 shows reaction BNR with PVC by influence ionized irradiation generates some decrease affinity copolymer to toluene compare initial polymer, as shown decrease value of
was found by graphic chart.The density of copolymers was determined by hydrostatic weighting method[15], based on the definition of the mass loss of sample immersed in the liquid, which is inert in relation to copolymers.The solubility of irradiated copolymers SKN-40PVC30 was qualitatively studied. The different solvents were tested. It was found that irradiated copolymers dissolve in solvents such as toluene, dichloroethane, solvent, methyl ethyl ketene, dioxane, chloroform. These dates show that obtained copolymer didn’t loose its ability to dissolution in the result of reaction, because their initial components nearby solubility and have the same solvents. But, as known, strictly scientific criteria dissolving ability of solvent is a change of Gibbs free enthalpy of solvent in system polymer-solvent include both sides of dissolve processes: enthalpy and entropy changes. Table 1 shows reaction BNR with PVC by influence ionized irradiation generates some decrease affinity copolymer to toluene compare initial polymer, as shown decrease value of 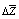 , for copolymer SKN-40+PVC-30, which contain 30% of PVC
, for copolymer SKN-40+PVC-30, which contain 30% of PVC 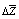 was counted by sorption isotherm (figure 1).
was counted by sorption isotherm (figure 1).
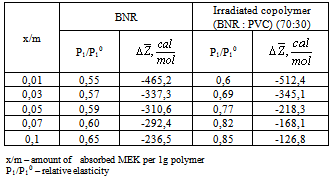
|
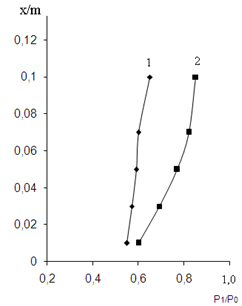 | Figure 1. The dependence of the relative steam tension of toluene/MEC (Р1/Р10) to the amount of absorbed toluene per g of polymer x / m. 1-BNR; 2- irradiated copolymer containing 30% PVC |
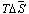 (figure 3) does not foster to solubility remaining negative as in initial polymer. The data presented above it is possible to can conclude that in solution of current copolymer defined role play energetic effect and small difference in change of thermo dynamical functions for copolymers in comparison initial polymer shows the absence of violent changes in solubility of initial polymer in the result of radiation-chemical reaction.
(figure 3) does not foster to solubility remaining negative as in initial polymer. The data presented above it is possible to can conclude that in solution of current copolymer defined role play energetic effect and small difference in change of thermo dynamical functions for copolymers in comparison initial polymer shows the absence of violent changes in solubility of initial polymer in the result of radiation-chemical reaction.  | Figure 2. Dependence of 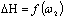 for systems MEK – BNR and MEC - irradiated copolymer containing 30% PVC for systems MEK – BNR and MEC - irradiated copolymer containing 30% PVC |
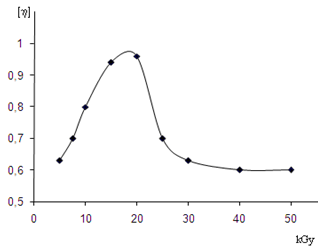 | Figure 3. Changing of characteristic viscosity (η) of solutions of irradiated copolymers BNR-PVC (in acetone), depend on by irradiation doses (kGy) |
|
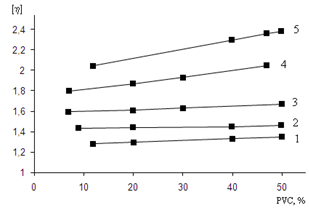 | Figure 4. The characteristic viscosity (η) of solutions of irradiated copolymers BNR-PVC (in acetone), obtained by irradiation doses (kGy): 1-10, 2-20, 3-30, 4-40, 5-50 |
4. Conclusions
- 1. In consequence of the thermodynamic research of solubility of the irradiated cross-linked copolymer of butadiene-nitrile rubber with PVC was founded that the scientific criterion of dissolvent power of the solvent is a changing of the solvent isobaric-isothermal potential in the polymer-solvent system, taking into consideration the dissolution process, i.e. enthalpy and entropy changes.2. In the dissolution of the polymer a defining role plays energy effect and the small difference in the changes of thermodynamic functions. For the copolymer compared to the original polymer, as a result of radiation-chemical reaction indicates the absence of abrupt changes in solubility.3. Using rheological studies for the study of molecular structural changes occur in the irradiated copolymer (BNR + PVC), gives an idea about character of change of solubility of the copolymers in acetone. Increasing of the absorbed dose due to a growth in the macromolecule chain mesh will lead to cross-linking and reduced solubility.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML of Methyl ethyl ketene by dissolving BNR and irradiated copolymers BNR-PVC
of Methyl ethyl ketene by dissolving BNR and irradiated copolymers BNR-PVC