-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2012; 2(5): 142-146
doi: 10.5923/j.materials.20120205.02
Nucleation Controlled in the Aggregative Growth of Strontium Carbonate Microcrystals
B. Sreedhar 1, Ch. Satya Vani 2, D. Keerthi Devi 1, V. Sreeram 3, M. V. Basaveswara Rao 3
1Inorganic and Physical Chemistry Division, Indian Institute of Chemical Technology (Council of Scientific and Industrial Research), Hyderabad, 500607 Andhra Pradesh, India
2Department of Chemistry, SR International Institute of Technology, Rampally, Keesara (M), RR District, 501301, Andhra Pradesh, India
3Department of Chemistry, Krishna University, Machilipatnam, 521001, Andhra Pradesh, India
Correspondence to: M. V. Basaveswara Rao , Department of Chemistry, Krishna University, Machilipatnam, 521001, Andhra Pradesh, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The influence of PABA(p-aminobenzoicacid) and HEEDTA (N-(2-hydroxyethyl) ethylenediamine- N, N, N- triacetic acid) on Strontionite crystals via simple CO2 diffusion route is described. The results showed that the experimental parameters have great influence on the shape evolution of products. The presence of templating species and varied pH are the key primary conditions for the growth morphology. Spike like crystals self assembled in the form of flower like and cauliflower shaped cluster with high crystallinity were identified. The crystals undergo an interesting morphology changes and have been characterized by X-ray diffraction (XRD), Scanning Electron Microscopy (SEM) and Fourier transform infrared spectroscopy (FT-IR) techniques.
Keywords: Biomaterials, Composite Materials, Crystal Growth, Electron Microscopy, p-amino Benzoic Acid, N-(2hydroxyethyl) Ethylenediamine-N, N’, N’’- Triacetic Acid
Cite this paper: B. Sreedhar , Ch. Satya Vani , D. Keerthi Devi , V. Sreeram , M. V. Basaveswara Rao , "Nucleation Controlled in the Aggregative Growth of Strontium Carbonate Microcrystals", American Journal of Materials Science, Vol. 2 No. 5, 2012, pp. 142-146. doi: 10.5923/j.materials.20120205.02.
Article Outline
1. Introduction
- The ability to manipulate the morphogenesis of materials through chemical synthesis is an important requirement of modern materials[1] chemistry, due to the fact that shape, dimension, and size of materials have great influence on their physico-chemical properties and their related applications. Among the variety of morphologies, self-assembled structures have been extensively studied due to their potential applications in various nanodevices[2,3]. However, only a few groups have examined synthetic methods that lead to assembled SrCO3 structures. Literature reports shows that, surfactants or templates, need to be disposed for obtaining pure sample[4,5]. Therefore, it is still a challenge to develop simple and reliable synthetic methods for the synthesis of self-assembled structures.Strontium carbonate (SrCO3) is widely used starting material of strontium for preparing a variety of strontium compounds,[6-8]. This has two traditional main applications. An additive in the production of glass for color television tubes and constituent of ferrite magnets[9,10]. In recent years, researchers have found additional applications of SrCO3 in other fields. Zhang and co-workers[11] discovered nanosized SrCO3-based chemiluminescence sensor showing high selectivity to ethanol and no response to foreign substances, such as gasoline,ammonia and hydrogen. Sreedhar et al synthesized SrCO3 hierarchical structure by natural materials[12] and organic additives[13]. Different SrCO3 hierarchical structures, such as flower -like[14], bundle-like, dumbbell-like, hexagonal star-like[15, 16], branch-like[17], especially spherical or sphere-like[14-16, 18,] rods, whiskers and ellipsoids,[19] fibers,[20] have been prepared using different methods, including reverse micelles,[21] solvothermal methods[22] liquid-liquid interfaces[23]. Though the methods are encouraging there is considerable scope for further study to improve the quality. SrCO3 microstructures, have attracted extraordinary attention due to their novel applications as sensors having chemilumenescence[24], catalyst[25, 26], color television tubes, chief constituent of ferrite magnets[27]. We are reporting the successful synthesis of SrCO3 structures of spike like and cauliflower shaped morphology, efficiently achieved by using two organic additives– PABA and HEEDTA with simple CO2 gas flow diffusion technique.
2. Experimental
2.1. Materials
- Para-aminobenzoicacid(C7H7O2N),N-(2hydroxyethyl) ethylenediamine-N,NI,NII-triaceticacid (C10H18O7N2), Ammonium carbonate (NH4)2CO3, sodium hydroxide (NaOH) and strontium chloride (SrCl2) were of analytical grade and used without further purification. Double distilled water was used in all experiments.
2.2. Characterization
- X-ray diffraction measurements of the strontium carbonate hierarchial structures were recorded using a Rigaku diffractometer (Cu radiation, λ = 0.1546 nm) running at 40 kV and 40 mA (Tokyo, Japan). FT-IR spectra of SrCO3 structures were recorded with a Thermo Nicolet Nexus (Washington, USA) 670 spectrophotometer. Crystals were collected on a round cover glass (1.2 cm), washed with deionized water and dried in a desiccator at room temperature. The cover glass was then mounted on a SEM stub and coated with gold for SEM analysis.
2.3. Preparation of SrCO3 Microcrystals
- A typical procedure for preparation of crystalline SrCO3 crystals was carried out as follows: In a glass bottle 2.5 mmol SrCl2, 0.1mmol PABA/HEEDTA were dissolved in 20 mL water and was stirred continuously to ensure complete solubility. Then the pH of the solution was adjusted to 7.0 and 10.0 by using 0.1M NaOH. After that the prepared solution was then covered with parafilm which was punched with three needle holes and placed in larger desiccator containing freshly crushed ammonium carbonate (20 g) at the bottom. After 24hr crystallization, the parafilm was removed and the white precipitate deposited on the glass bottle centrifuged and washed thoroughly with distilled water, followed by ethanol and allowed to dry at room temperature for further crystallization.
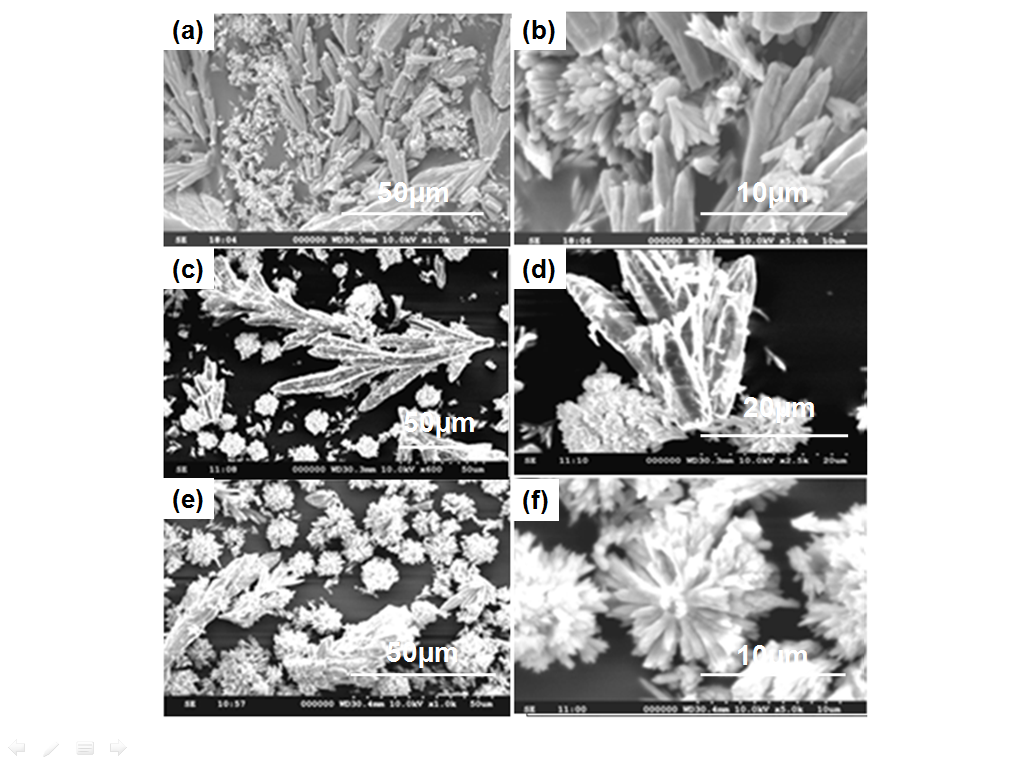 | Figure 1. SEM images of SrCO3 in the presence of PABA at varied pH conditions (a,b) pH 3.0, (c, d) pH 7.0, and (e, f) pH 10.0 |
 | Figure 2. SEM images of SrCO3 in the presence of HEEDTA at varied pH conditions (a, b) initial pH 3.0, (c, d) pH 7.0, and (e, f) pH 10.0 |
3. Results and Discussion
3.1. Effect of Additives on the Morphology of SrCO3
- Significant changes in the morphologies were observed when the pH of the reaction conditions varied between 7 and 10. Figure 1 and 2 shows the SEM images of SrCO3 structures by adding the additives PABA and HEEDTA at different pH conditions. In general SrCO3 dendrimers are obtained in the absence of additive[12], while in the presence of PABA at initial pH 3.0, individual spike like crystals were observed (Figure 1a, b). When the pH of the reaction mixture was increased to 7, the individual spikes aggregates to coniform like structures (Figure 1c, d). On further increasing the pH to 10.0, the spikes aggregates more closely and self assembled into flower like structures (Figure 1e, f). Figure 2a shows the SEM images of SrCO3 structures in the presence of the additive HEEDTA. Remarkable changes in the morphology was observed with this additive and the shape of SrCO3 structures changed from dendrimeric to cauliflower bunch like structures with short spikes of subunits having smooth surface at initial pH 3 (Figure 2 a, b). At neutral pH mixed phase of cauliflower bunches with rough surface and spadix shaped structures with fibre like units are identified (Figure 2c, d). At pH 10.0, morphology of the SrCO3 structures appears similar to that observed at pH 3 with rough surface (Figure 2 e, f). The strontianite crystals clearly aggregates into cauliflower bunch like and coniform structures in CO2 diffusion route. To the best of our knowledge, such close packed aggregates of strontionite crystals have not been observed in other biomimetic approaches to the growth of SrCO3 crystals.
3.2. Structural Characterization of SrCO3 Microcrystals
- The XRD pattern of the isolated solids could be indexed to that orthorhombic structure of strontium carbonate and all the peaks are assigned by using JCPDS (05-418). The results implied that the aggregation did not alter the phase structure of SrCO3.To our expectation, the crystallinity nature of the product was sensitive to that pH conditions. At lower pH condition showed a greater crystallinity than the product prepared under higher pH condition, implying the influence of solution pH on the crystallinity. In Figure 3 the pattern of SrCO3 crystals obtained in aqueous solution displays the following diffraction peaks with (hkl) indices (110), (111), (021), (002), (121), (200), (130), (220), (040), (032), (041), (202), (132), (141) respectively. Similarly in figure 4, the diffraction peaks with (hkl) indices (110), (111), (021), (002), (121), (200), (112), (220), (032), (040), (132), and (113), of pure orthorhombic strontionite respectively. It may also be seen that the peak (111) is the strongest, suggesting that SrCO3 crystals obtained in aqueous solution grow mainly along with the (111) phase.
3.3. FTIR Studies
- To identify the growth mechanism and the effect of PABA and HEEDTA on SrCO3 microstructures, the sample was analyzed by FT-IR spectroscopy. The sharp peaks at 856, 703 cm-1 (Figure 5 a), 855, 701 cm-1 (Figure 5 b) and 857, 701 cm-1 (Figure 5 c) are in-plane and out-plane bending of CO32-.
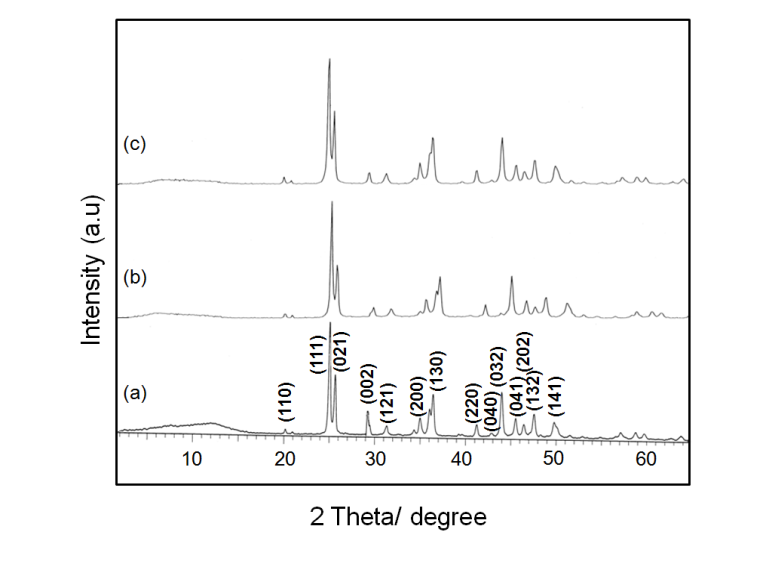 | Figure 3. XRD pattern of SrCO3 in the presence of PABA (a) initial pH 3.0, (b) pH 7.0, and (c) pH 10.0 |
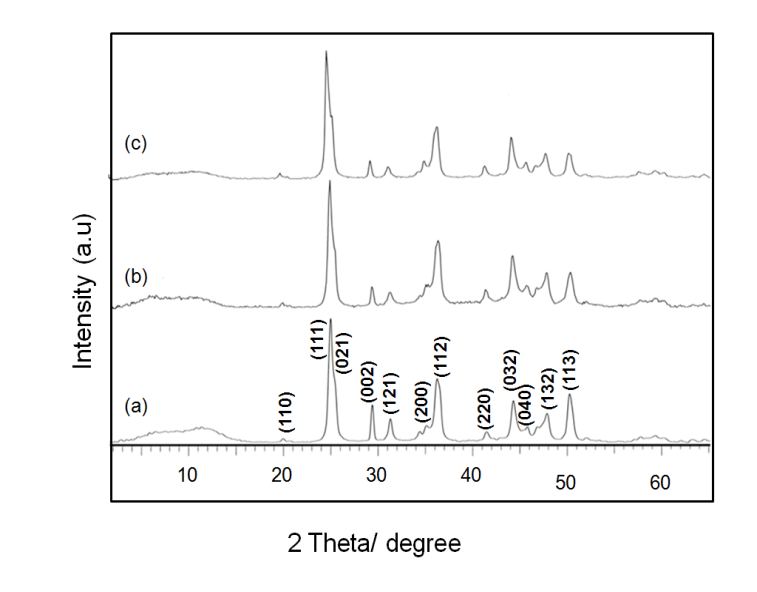 | Figure 4. XRD pattern of SrCO3 in the presence of HEEDTA (a) initial pH 3.0, (b) pH 7.0, and (c) pH 10.0 |
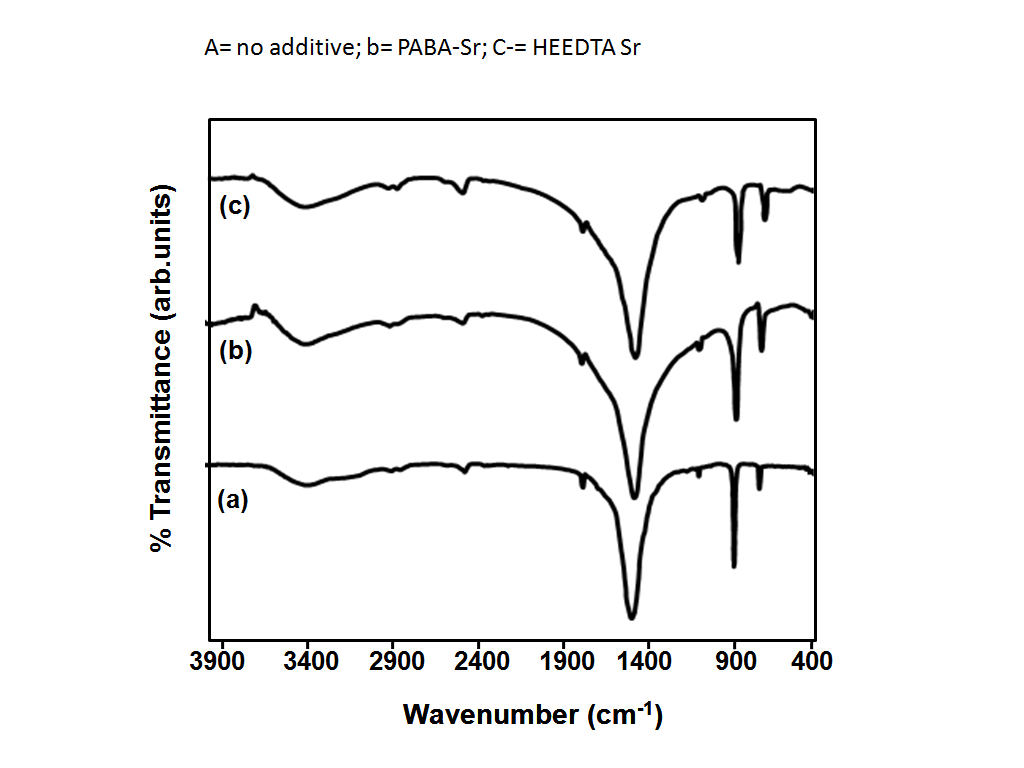 | Figure 5. FT-IR of SrCO3 microstructures nucleated (a) in the absence of an additive, (b) presence of PABA and (c) presence of HEEDTA |
4. Conclusions
- In summary, uniform hierarchical SrCO3 complex structures in the form of spike like and cauliflower bunch like units were efficiently obtained by a facile ammonium carbonate method in the presence of PABA and HEEDTA as additives. The present uniform hierarchical SrCO3 structures were self assembled by the related crystalline particles, assisted by the additives would enlarge the potential applications of strontium carbonate microstructures. This strategy may also be expected for the preparation of other metal carbonates.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML