-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2012; 2(4): 119-124
doi: 10.5923/j.materials.20120204.04
Synthesis and Characterization of ZrO2 Thin Films
L. P. Borilo , L. N. Spivakova
Tomsk State University, Tomsk, Russia
Correspondence to: L. P. Borilo , Tomsk State University, Tomsk, Russia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Zirconia thin films with thicknesses of 40-120 nm on glass, single-crystal silicon, quartz, polycor, and sapphire substrates have been prepared from zirconium oxochloride and ethanol FFSs. The physicochemical processes involved in film formation and the phase composition and properties of the films have been studied. The films prepared on glass or quartz are amorphous; those on silicon, polycor, or sapphire have a crystal structure. The resulting ZrO2 films have refractive index indicator 1,86 – 2,08, are insulators, with high indicators of bandgap width 5,0 – 5,2 eV, absorption edge is limited by 220 nM, which allows to use it as reallot light covering.
Keywords: Sol-Gel Technology, Thin Films, Zirconium Dioxide
1. Introduction
- Films materials play a special role in the development of civilizations based on high technologies. Studying optical characteristic of dielectric resulted in using thin layer as reallot light covering and interference filters. Among different material classes oxides have various functional electro physical properties (electro physical, optical, mechanical etc), which allows to use these materials as a basis for synthesis of many film materials. At the present stage of development of photonics, optoinformatics, optotechnics and laser optics the requirements to optics properties of films extend[1-2].The most challenging issue is the absence of transparent in broad spectral zone film-forming materials with high Refractive index. Zirconia-based thin films have a high potential. Zirconia is transparent in the visible; it has high refractive index and bandgap values, good adhesion to substrates (glass, ceramics, silicon, polycor, and sapphire), thermal stability, and corrosion resistance[3-6].Sol-gel technology, which is a synthetic method involving chemical condensation in a liquid phase, is regarded as the most efficient and simplest method for manufacturing nanoparticles. This method can provide not only dispersed powders but also thin films based on complex chemical systems having layer thicknesses of 10 to 200 nm and multilayer films having thicknesses up to 1 μm. The synthesis and characterization of nanocrystalline zirconia-based powders prepared by various methods are found in periodicals[7-12]. To use thin films materials based on dioxide zirconium in optics, particularly as reallot light covering, we should studythe influence of conditions of synthesis on thickness, phase composition and structure of resulting films, evaluate the influence of a substrate (glass, quartz, etc) on physicochemical properties of the films.Therefore, here we study the physicochemical processes involved in the preparation of ZrO2 thin films on various substrates by sol-gel technology from film-forming solutions (FFSs) and the phase composition, structure, and properties of these films as dependent on the synthesis parameters.
2. Experimental Work
- Thin films were prepared by sol-gel technology from FFSs. The FFSs were prepared from 96% ethanol and zirconium oxochloride ZrOCl2·8H2O (pure for analysis); the solution concentration was 0.4 mol/L. Films were prepared on glass, single-crystal silicon, quartz, polycor, and sapphire using centrifugation at 4000 rpm or pulling at 5 mm/s and subsequent heat treatment at 60℃ for 30 min and at 600-1000°C for 1 h.The film-forming power of solutions as a function of aging time was studied viscometrically on a glass capillary viscometer (the capillary diameter was 0.99 mm; temperature was 25℃). Reduced viscosity ηsp/c was determined as a function of zirconium oxochloride concentration by diluting the FFS with ethanol; oxochloride concentrations ranged within 0.5- 3.3 g/dL. The concentration dependences were processed after Bogoslovskii et al.[13].The thermolysis of the dried FFS was studied on a Q-1500 derivatograph (25-1000℃, calcined A12O3 as reference, air, heating at 10 K/min, Alundum crucibles). A microbalance based on a quartz piezocrystal resonator was used in the thermoanalytical experiments; the weighing accuracy was 10-8 g[14]. IR absorption spectra were recorded for films on KBr substrates annealed at various temperatures within 400-4000 cm4 on a Perkin-Elmer Spectrum One spectrometer. The composition of films was determined on a DRON-3M diffractometer (CuKα radiation, λ = 1.5418 nm, Ni filter). The refractive index and film thickness were determined on an LEF-3M laser diffractometer. The optical parameters were calculated using the uniform nonabsorbing layer on an isotropic substrate model[15]. Reflection andtransmission spectra in the visible and IR were recorded on SF-20 and Perkin-Elmer Spectrum One spectrometers. Dielectric constants were calculated by Kramers-Kroning relations from the reflection and transmission spectra. The bandgap width was calculated from the absorption edge position. Adhesion was determined by scleroscopy on a PMT-3 microhardness tester.
3. Results and Discussion
- The solutions from which thin films can be deposited by sol-gel technology should conform to several requirements. When precursors are dissolved in the solvent, some period is needed for so-called solution ripening or sol formation. This period ranges from several minutes to several days, depending on the precursor. At this stage, the true solution transforms to a colloidal one because of solvation, hydrolysis, complex formation, and condensation. The resulting associations are capable of being anchored to the surface when the solution is applied to the substrate.We used viscosity as a measure of the film-forming power of the solution[16-18]. Solution viscosities were plotted as function of time and possibility of film preparation from these solutions. Our studies showed that a fresh zirconium oxochloride-based ethanolic solution has not film-forming properties. The film-forming ability appears 2-3 days after the solution was prepared. The solution viscosity increases strongly during this period because of the solvation of zirconium oxochloride and the formation of hydroxo complexes[Zr4(ОН)8(С2Н5ОH(Н2O)16-х]С18; as a result, a stable sol is formed in the solution. Good films with reproducible properties can be prepared if the solution viscosity reaches 2.3 × 10-3 Pa s.The processes occurring in the FFS during storage and operation change the composition of the complex: the number of molecules of water of coordination decreases, more OH groups appear linked to zirconium, some water molecules are displaced from the inner coordination sphere, and the hydroxo cation charge decreases:[Zr4(OH)8(C2H5OHUH2O)16-х]Cl8 →[Zr4(OH)12(C2H5OH)x(H2O)12x]Cl4 + 4HC1,[Zr4(OH)12(C2H5OH)x(H2O)12-x]Cl4 →[Zr4 (ОH)16(С2Н5ОH)x(H2O)8-x] + 4HC1,[Zr4(OH)8(H2O)16-x(C2H5OH)J8+ →[Zr4(OH)8+y(H2O)16 -x -у(С2Н5ОH)x](8 -y)+ + yH+.As a result, the system loses stability because of coagulation, and the solution viscosity increases dramatically and exceeds 3.5 × 10-3 Pa s. The films prepared from such solutions have nonuniform thicknesses and low refractive indices. Thus, the film-forming properties of the solution (the ability to form films) exist within a limited period time, namely, while the solution is ripening and aging. The optimal viscosities of zirconium oxochloride-based solutions for preparing ZrO2 films are (2.3 to 3.5) 3.5 × 10-3 Pa s.The zirconium oxochloride-based FFS being a solution based on a polymeric hydroxo complex, Kolen'ko et al.' approach[12] is suitable for the quantitative description of colloid interactions in this solution. In this context, we studied the viscosity of the zirconium oxochloride-based FFS as a function of dilution and calculated the covalent and electrostatic terms of the Gibbs free energy of mixing. To describe reduced viscosity ηsp/c as a function of concentration, we used a linear extrapolation equation, namely, Huggins's equation
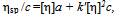 | (1) |
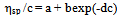 | (2) |
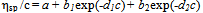 | (3) |
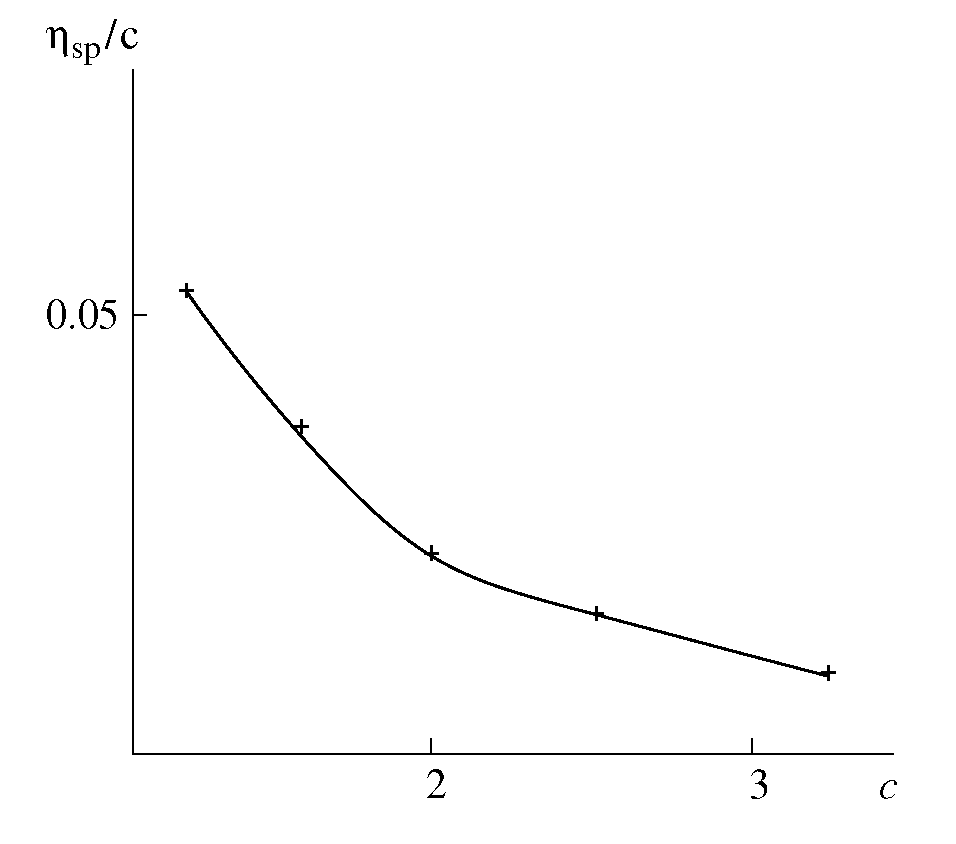 | Figure 1. Reduced viscosity ηsp/c for the zirconium oxochloride-based FFS vs. zirconium oxochloride concentration с (g/dL) |
|
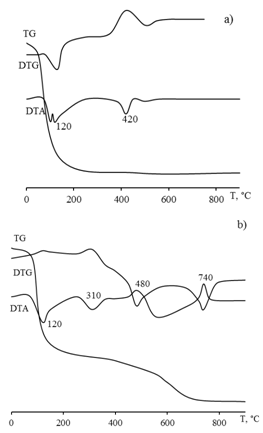 | Figure 2. Data of differential thermal analysis а) Zirconium oxochloride powder b) Powder prepared from film-forming solutions based on zirconium oxochloride |
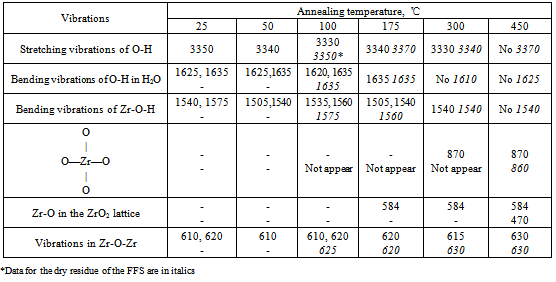
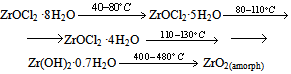 The thermolysis of the powder prepared from the FFS differs in that it contains the product of zirconium oxochloride thermolysis:
The thermolysis of the powder prepared from the FFS differs in that it contains the product of zirconium oxochloride thermolysis: 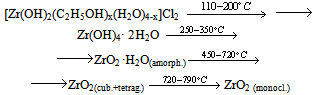 ZrO2 film formation is a more intricate process, as shown by piezocrystal weighing, IR spectroscopy, and X-ray powder diffraction. This process has the following distinguishing feature: when the solution is applied to the substrate, chloride ions remain in the solution and are not contained in the thin FFS layer on the substrate surface. The solution is anchored to the substrate via the interaction of zirconium hydroxo complexes with the surface hydroxide groups of the substrate as a result of hydrolysis. Schematically, subsequent dehydration in a thin layer can be represented as follows:
ZrO2 film formation is a more intricate process, as shown by piezocrystal weighing, IR spectroscopy, and X-ray powder diffraction. This process has the following distinguishing feature: when the solution is applied to the substrate, chloride ions remain in the solution and are not contained in the thin FFS layer on the substrate surface. The solution is anchored to the substrate via the interaction of zirconium hydroxo complexes with the surface hydroxide groups of the substrate as a result of hydrolysis. Schematically, subsequent dehydration in a thin layer can be represented as follows: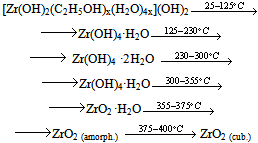 The IR spectra of a freshly applied film contain peaks corresponding to zirconium hydroxide and physisorbed water (Table 3). Film adhesion to the substrate is low; the refractive index is about 1.6, which is not characteristic of zirconia (Fig. 3). The refractive index of the film systematically increases during heat treatment as temperature increases, while the film thickness decreases to reach a steady-state value at 400℃ (Fig. 3). The values of 2.0-2.1 for the refractive index mean that the film composition corresponds to zirconia.
The IR spectra of a freshly applied film contain peaks corresponding to zirconium hydroxide and physisorbed water (Table 3). Film adhesion to the substrate is low; the refractive index is about 1.6, which is not characteristic of zirconia (Fig. 3). The refractive index of the film systematically increases during heat treatment as temperature increases, while the film thickness decreases to reach a steady-state value at 400℃ (Fig. 3). The values of 2.0-2.1 for the refractive index mean that the film composition corresponds to zirconia.
| ||||||||||||||||||||||||||
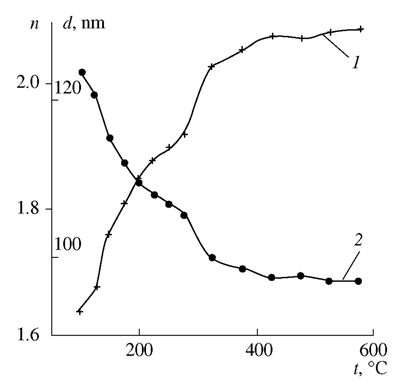 | Figure 3. (1) Refractive index and (2) thickness vs. temperature for ZrO2 films |
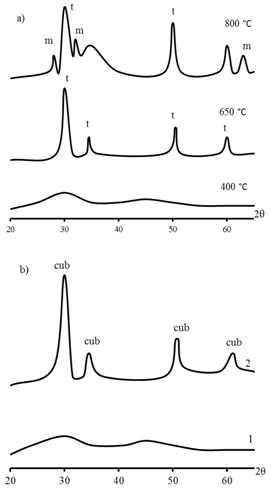 | Figure 4. Data of X-ray powder diffraction a)ZrO2 powder b) ZrO2 thin 1 – on glass; 2 – on quartz |
|
| ||||||||||||||||||||||||||||||||||||||||||||
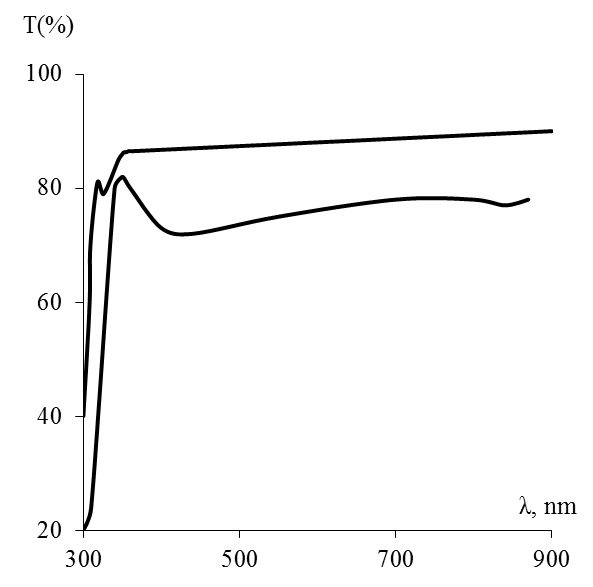 | Figure 5. Transmittance spectrum T (%) depending on wave length λ, nm 1-Quartz; 2- ZrO2 thin film on quartz |
4. Conclusions
- Zirconia thin films with thicknesses of 40-120 nm on glass, single-crystal silicon, quartz, polycor, and sapphire substrates have been prepared from zirconium oxochloride and ethanol FFSs. The physicochemical processes involved in film formation and the phase composition and properties of the films have been studied. The film-forming power of the FFS is controlled by the formation of zirconium hydroxo complexes in the solution; the optimal viscosities of FFSs for the deposition of quality films are within 2.3-3.5 × 10-3 Pa s. Thermally induced zirconia formation occurs in several stages involving removal of solvolysis products and ZrO2 crystallization. The films prepared on glass or quartz substrates are amorphous; those on silicon, polycor, or sapphire have a crystal structure depending on the annealing temperature and film thickness. The resulting ZrO2 films have refractive index indicator 1,86 – 2,08, are insulators, with high indicators of bandgap width 5,0 – 5,2 eV, absorption edge is limited by 220 nM, which allows to use it as reallot light covering. ZrO2 films have high refractive indices, are insulators, and have good adhesion to substrates.
References
| [1] | V. I. Vereshchagin, V. V. Kozik, L. R Borilo, et al., Poly-funcitonal Inorganic Materials based on Natural and Artifical Compounds (Izd-vo Tomsk. Univ., Tomsk, 2002)[in Russian]. |
| [2] | L. R Borilo, Thin-Film Inorganic Nanosystems (Izd-vo Tomsk. Univ., Tomsk, 2003)[in Russian]. |
| [3] | N.T. Soo, N. Prastomo, A Matsuda, G. Kawamura, H. Muto, A.F. Mohd Noor, Z. Lockman, K.Y. Cheong, Applied Surface Scince 258 (2012), 5250-5258 |
| [4] | J. Mosa, O. Fontaine, P. Ferreira, R. P. Borges, et al. Electrochimica Acta 56 (2011), 7155-7162 |
| [5] | H. Bensouyad, H. Sedraty, H Dehdouh, M.Brahimi, et al. Thin solid films 519 (2010), 96-100 |
| [6] | K. Joy, S. Lakshmy, Prabitha B. Nair, Georgi P. Daniel. Journal of Alloys and Compounds 512 (2012),149-155 |
| [7] | V. F. Petrunin, V. V. Popov, Khunchzhi Chzhu, and A. A. Timofeev, Neorg. Mater. 40 (3), 303 (2004). |
| [8] | N. A. Shabanova, V. V. Popov, and P. D. Sarkisov, Chem-istry and Technology of Nanodisperse Oxides (Aka-demkniga, Moscow, 2006)[in Russian]. |
| [9] | N. N. Oleinikov, I. V. Pentin, G. P. Murav'eva, and V.A. Ketsko, Zh. Neorg. Khim. 46 (9), 1413 (2001)[Russ. J. Inorg. Chem. 46 (9), (2001)]. |
| [10] | V. I. Pentin, N. N. Oleinikov, G. P. Murav'eva, et al., Neorg. Mater. 38 (10), 1203 (2002). |
| [11] | L. G. Karakchiev, E. G. Avvakumov, О. B. Vinokurova, et al., Zh. Neorg. Khim. 48 (10), 1589 (2003)[Russ. J. Inorg. Chem. 48 (10), (2003)]. |
| [12] | Yu. V. Kolen'ko, A. A. Burukhin, and B. R. Churagulov, Zh. Neorg. Khim. 47 (11), 1755 (2002)[Russ. J. Inorg. Chem. 47(11), (2002)]. |
| [13] | A. Yu. Bogoslovskii, E. G. Pribytkov, and G. A. Teren'eva, Zh. Prikl. Khim. 71 (2), 294 (1998). |
| [14] | V. V. Serebrennikov, G. M. Yakunina, V. V. Kozik, and A. N. Sergeev, Rare-Earth Elements and Their Compounds in Electronic Engineering (izd-vo Tomsk. Univ., Tomsk, 1980)[in Russian]. |
| [15] | В. M. Komranov and В. A. Shapochnykh, Measurements of Parameters of Optical Coatings (Mashinostroenie, Moscow, 1986)[in Russian]. L. P. Borilo, V. V. Kozik, N. A. Skorik, and V. V. Dyukov, Zh. Neorg. Khim. 40 (10), 1596 (1995). |
| [16] | R. V. Gryaznov, L. P. Borilo, V. V. Kozik, and A. M. Shul'pekov, Neorg. Mater. 37 (7) |
| [17] | V. V. Kozik, L. P. Borilo, and A. M. Shul'pekov, Zh. Prikl. Khim. 73 (11), 1872 (2000). |
| [18] | M. B. Fialko, Nonisothermal Kinetics in Thermal Analysis (Izd-vo Tomsk. Univ., Tomsk, 1981)[in Russian]. |
| [19] | V. V. Kozik, A. M. Shul'pekov, and L. P. Borilo, Izv. Vyssh. Uchebn. Zaved., Fiz., No. 12, 77 (2002, 828 (2001). |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML