-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2012; 2(4): 105-109
doi: 10.5923/j.materials.20120204.02
Biomimetic Mineralization of BaCO3 Microstructures By Simple CO2 Diffusion Method
B. Sreedhar 1, Ch. Satya Vani 2, D. Keerthi Devi 1, V. Sreeram 3, M. V. Basaveswara Rao 3
1Inorganic and Physical Chemistry Division, Indian Institute of Chemical Technology (Council of Scientific and Industrial Research), Hyderabad, Andhra Pradesh, 500607, India
2Department of Chemistry, SR International Institute of Technology, Rampally, Keesara (M), RR District, Andhra Pradesh, 501301, India
3Department of Chemistry, Krishna University, Machilipatnam, Andhra Pradesh, 521001, India
Correspondence to: M. V. Basaveswara Rao , Department of Chemistry, Krishna University, Machilipatnam, Andhra Pradesh, 521001, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Barium carbonate (BaCO3) microstructures have been synthesized in aqueous solution under ambient conditions with PABA (p-amino benzoic acid) and HEEDTA (N-(2 hydroxyethyl) ethylenediamine-N, N’, N’’- triacetic acid) as simple additives. In this study we demonstrate that the integration of both the additives, PABA and HEEDTA under different experimental conditions, such as crystallization sites and pH will extend the possibilities for controlling the shape and size on microstructures of the inorganic crystals by means of a slow CO2 simple diffusion route. The influence of variation of pH condition with two different additives on the particle size and morphology was investigated. Scanning electron microscopy, Fourier transform infrared spectroscopy and X-ray powder diffractometry were used to characterize the products. The results indicate that bunch like dendritic and limpet teeth shaped, BaCO3 microstructures were obtained. Increasing pH led to the separation of rods from the complex structures.
Keywords: Barium Carbonate, P-Amino Benzoic Acid, N-(2hydroxyethyl) Ethylenediamine-N, N’, N’’- Triacetic Acid and Biomineralization
Article Outline
1. Introduction
- Highly ordered complex structures have been studied extensively due to their unique nature and fantastic properties different from those of the monomorph structures[1]. For this, biomimetic synthesis of inorganic materials with complex and heirarchial structures, templates or organic additives with complex functionalization patterns are used to control the nucleation growth and alignment of inorganic crystals.Researchers are increasingly concerned with the synthesis of advanced materials with enhanced properties. The use of inorganic-organic interface for the morphosynthesis of inorganic materials is an emerging soft chemical route[2]. The molecular interactions between inorganic - organic interface seem to control nucleation and growth which often stabilizing new modifications and morphologies[3]. Carbonate minerals like CaCO3, BaCO3, and SrCO3, were intensively studied as a model compound for biomimetic mineralization[4]. BaCO3 is having close resemblance with the aragonite type mineral with many industrial applications in the ceramic and glass industries as well as its use as a precursor for magnetic ferrites and/or ferroelectric materials[5].Barium carbonate (BaCO3) is also used as aprecursor for producing superconductor and ceramic materials[6] and other important applications in optical glass and electric condensers[7]. Various morphologies such as helical BaCO3 fibers[8,9] candy-like, needle like or olivary like[10], nanofibers and rod like structures[11] in the presence of different additives are reported. In literature different biomimetic approaches to control the morphology of carbonate systems using a variety of additives/templates, such as Langmuir films[12-14], ultrathin organic films[15], self assembled monolayers[16-18], varied soluble additives like synthetic peptides[19], dendrimers[20,21], nicotinic acid[22], H5hpdta, H3heidi[23], PABA[24] and common polymers[25,26] have been described. Here in, we present a study, on the biomimetic synthesis of BaCO3 crystals by using two different organic additives, namely PABA and HEEDTA with simple CO2 gas slow diffusion technique to prepare dendritic and limpet teeth shaped BaCO3 structures, respectively in aqueous solution. However, to the best of our knowledge, limpet–teeth like BaCO3 structures have not been reported till now.
2. Experimental
2.1. Materials and instruments
- Para-aminobenzoicacid (C7H7O2N), N-(2hydroxyethyl) ethylenediamine-N, N’, N’’-triaceticacid (C10H18O7N2), ammonium carbonate (NH4)2CO3, sodium hydroxide (NaOH) and barium chloride (BaCl2) were of analytical grade and used without further purification. Double distilled water was used in all experiments.
2.2. Materials
- X-ray diffraction measurements of the barium carbonate hierarchial structures were recorded using a Rigaku diffractometer (Cu radiation, λ = 0.1546 nm) running at 40 kV and 40 mA (Tokyo, Japan). FT-IR spectra of BaCO3 structures were recorded with a Thermo Nicolet Nexus (Washington, USA) 670 spectrophotometer. The crystals were collected on a round cover glass (1.2 cm), washed with deionized water and dried in a desiccator at room temperature. The cover glass was then mounted on a SEM stub and coated with gold for SEM analysis.
2.3. Synthesis of BaCO3 Crystals
- A typical procedure for preparation of crystalline BaCO3 crystals was carried out as follows: 2.5 mmol BaCl2, 0.1 mmol PABA/HEEDTA were dissolved in 20 mL H2O, prepared in a glass bottle and stirred continuously for complete dissolution. Then the pH of the solution was adjusted to 7.0 and 10.0 by using dilute NaOH. After that the prepared solution was then covered with parafilm which was punched with three needle holes and placed in larger desiccator containing crushed ammonium carbonate at the bottom. After 24hr crystallization, the parafilm was removed and the white precipitate deposited on the glass bottle rinsed with distilled water and ethanol and allowed to dry at room temperature for further crystallization.
3. Results
3.1. Structural Characterization of BaCO3 Crystals
- The crystal structures and the phase purity of the materials were determined by X-ray diffraction (XRD). XRD patterns of the as-prepared dendritic and limpet teeth shaped BaCO3 microstructures at two different pH conditions – 7 and 10 are presented in Figures 1 and 2. All the observed diffraction peaks of the products can be attributed to pure orthorhombic BaCO3 crystals (JCPDS card number: 71-2394). In Figure 1, the pattern of BaCO3 crystals obtained in PABA solution displays the following diffraction peaks with (hkl) indices (110), (020), (111), (021), (002), (112), (200), (220), (221) (041), (202), (132), and (113). In Figure 2, the pattern of BaCO3 crystals obtained in HEEDTA solution displays the following diffraction peaks with (hkl) indices (110), (020), (111), (021), (002), (112), (200), (220), (221), (132), and (113), of pure orthorhombic witherite phase. It may also be seen that the peak at (111) is the strongest, suggesting that BaCO3 crystals obtained with PABA and HEEDTA aqueous solutions were well oriented and grew mainly along the crystallographic C-axis. This result was also maintained by SEM observation, which exhibited the dendrite and limpet teeth morphology of complex hierarchical structures. All of these complex microstructures were composed of microrods with diameters in the range of 1μm – 1.5μm. In comparison with Figures 1 (a)-(c), the pattern in Figures 2 a-2c has three changes. One is that two new diffraction peaks, (041) and (202) faces appear, and the second is that the relative intensity of (112), (200) planes are decreased at initial pH 3 and raised pH 10. The third main difference is the resolution of (021) as separate peak next to (111). This suggests that PABA and HEEDTA have different influence on the crystal growth of BaCO3 which also can be interpreted by the fact that the two organic molecules can adsorb onto certain crystal faces BaCO3 crystals and influence the crystal growth process.
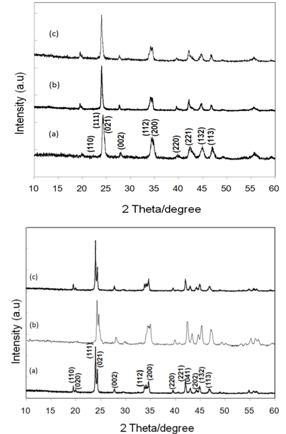 | Figure 1. XRD pattern of BaCO3 in the presence of PABA 1 (a) pH 3.0, (b) pH 7.0 and (c) pH 10.0. Figure 2.XRD pattern of BaCO3 in the presence of HEEDTA 2 (a) pH 3.0; (b) pH 7.0, and (c) pH 10.0 |
3.2. Influence of Additives on the Morphology of BaCO3
- Significant changes in the morphologies were observed when the pH of the reaction conditions were varied – 7 and 10. Figure 3 and 4 show the SEM images of BaCO3 structures obtained before and after adding the additives PABA and HEEDTA, respectively at initial pH(3.0) and rised pH(7.0 and 10.0) conditions. As can be seen From Figure 3a, and 4a, BaCO3 microrods are obtained in the absence of additive, while in the presence of PABA at starting pH 3.0(without rising with NaOH), bunch like dendritic structures were observed (Figure 3b). From the magnified image it can be clearly seen that the branch like product are built up of much smaller size subunits shown as inset in Figure 3b. No changes were observed in the morphology when the pH of the reaction mixture was increased to 7, except an increase in the length of the dendritic structures as shown in Figure 3c. On further increasing the pH to 10.0, the bunch like dendritic structures are separated and become almost twin shaped rods. As can be seen, at all the three different pH conditions, similar morphological changes with variation in the length of the dendritic structures were identified. Figure 4 shows the SEM images of BaCO3 structures in the presence of the additive HEEDTA. Significant changes in the morphology was observed with this additive and the shape of BaCO3 structures changed from rods to limpet teeth which is isostructural with the mineral aragonite, with sizes ranging from several micrometers to several tens of micrometers. At initial pH 3.0 more branches with long rods and sharp tips are observed. At neutral pH, short sized rods with less number of branches that are dispersed are seen. At pH 10.0, morphology of the BaCO3 structures appears similar to that observed at initial pH 3. Moreover, the tips of the rods are not too sharp as seen in initial pH 3.0. So it can be concluded that, even though the morphology at different pH remain same, variation in the branching and size of the subunits is observed in the controlled experiments through the slow gas diffusion method.
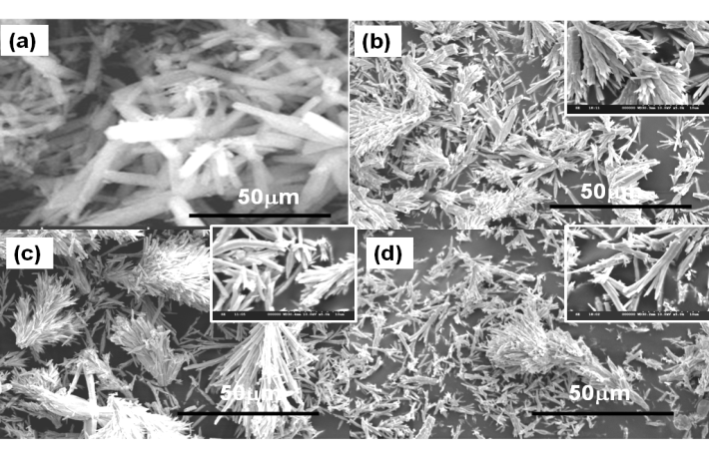 | Figure 3. SEM images of BaCO3 in the presence of PABA at varied pH conditions (a) absence of PABA, (b) initial pH 3.0, (c) pH 7.0, and (d) pH 10.0. |
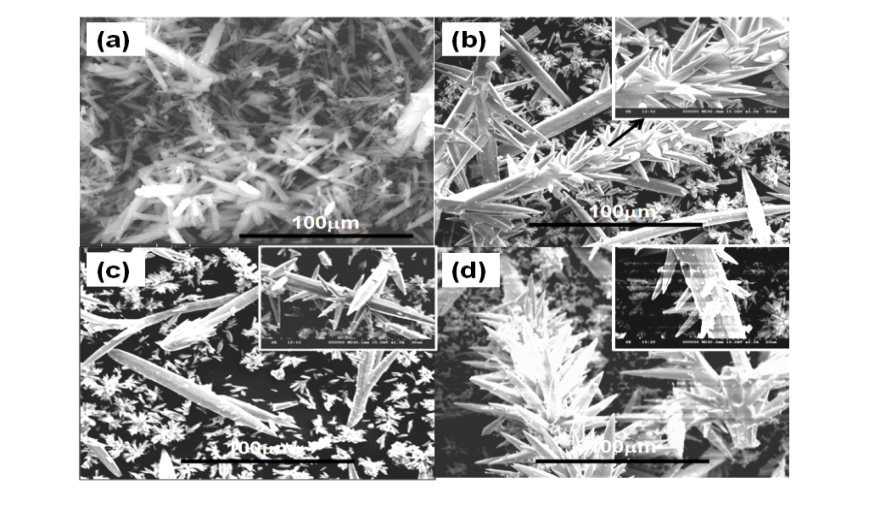 | Figure 4. SEM images of BaCO3 in the presence of HEEDTA at varied pH conditions (a) absence of HEEDTA, (b) initial pH 3.0, (c) pH 7.0, and (d) pH 10.0. |
3.3. FT-IR Analysis
- To identify the growth mechanism and the effect of PABA and HEEDTA on BaCO3 microstructures, the sample was analyzed by FT-IR spectroscopy. The IR bands at 1445 cm-1 (Figure 5b), and 1435 cm-1 (Figure 5c) correspond to the asymmetric stretching mode of C-O bond, while the weak band at 1059 cm-1 (Figure 5b, c) is attributed to the symmetric C-O stretching vibration. The weak bands at 1165 and 1059 cm-1 are C-H in-plane bending vibrations and the small peaks between 1500 cm-1 to 1400 cm-1 are C-C stretching vibrations in aromatic ring (Figure 5b). The extra peaks in Figure 5b and 5c are attributed to the functional groups (-COOH, NH2, OH) that are present in the additives. In comparison with Figures 5b, the C-O stretching vibration peak around 1435 cm-1 in Figure 5c, shifts to lower frequency by 10 cm-1 (1445 cm-1), suggesting that PABA and HEEDTA have different influence of BaCO3. This is probably due to the fact that the two organic molecules can adsorb onto the different planes of BaCO3 nuclei and influence the mode of crystal growth, resulting in little change of microstructure.
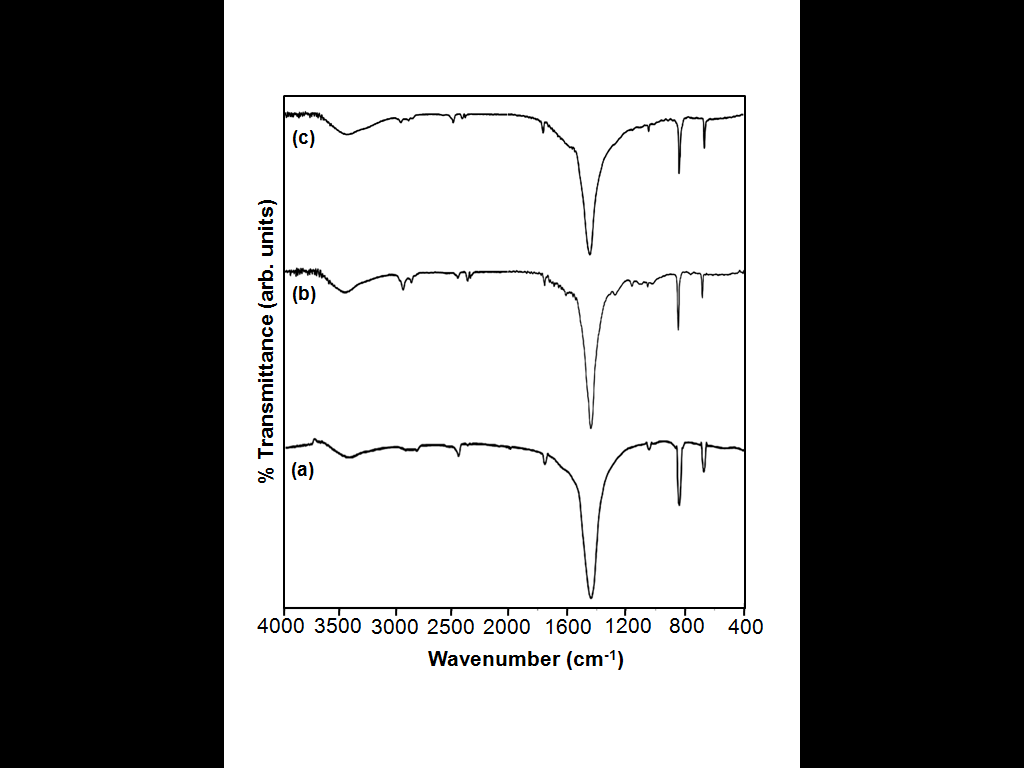 | Figure 5. FT-IR of BaCO3 microstructures nucleated (a) in the absence of an additive, (b) presence of PABA, and (c) presence of HEEDTA. |
4. Discussion
- On the basis of SEM observation, the growth mechanism for the generation of BaCO3 complex structures can be described as rod to dendritic like structures and rod to limpet teeth shaped progression for PABA and HEEDTA, respectively. The first stage is the nucleation process that is the initial reaction between Ba2+ and amino/ carboxylic anions in PABA and hydroxyl/carboxylic anions in HEEDTA, which generates the BaCO3 nuclei. The second stage is the formation of BaCO3 rods via orientation growth along the crystallographic c-axis, as demonstrated by XRD. The third stage involves the formation of branches at the ends of the primary rod in PABA whereas with HEEDTA all over the branches on the primary rod leading to the formation of bunch like dendritic structures or limpet teeth like BaCO3 microstructures. The results above show that PABA promotes the formation of branch like dendritic structures while HEEDTA favors the formation of limpet teeth like BaCO3 crystals. So it can be presumed that the amino and/or carboxylic anions in PABA and hydroxyl and/or carboxyl anions in HEEDTA control the formation of microstructures by adsorbing onto certain facets of BaCO3 crystals.
5. Conclusions
- Different morphologies of BaCO3 complex structures were controlled by the cooperation of the PABA and HEEDTA additives by means of a slow CO2 simple diffusion technique. It is noticeable that the pH variation with both the different organic additives has remarkable effect on the morphology. In this system pH and additive is the key factor for the biomineralisation research and development of new materials which could find various applications.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML