-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2012; 2(3): 82-86
doi: 10.5923/j.materials.20120203.09
Synthesis of Gold-Palladium Bimetallic Nanoparticles and Surface Catalytic Activity in Suzuki Coupling Reactions Using in FTIR Spectroscopy
P. Venkatesan, J. Santhanalakshmi
Department of Physical Chemistry, University of Madras, Guindy Campus, A.C.Tech., Chennai Tamil Nadu, 600025, India
Correspondence to: J. Santhanalakshmi, Department of Physical Chemistry, University of Madras, Guindy Campus, A.C.Tech., Chennai Tamil Nadu, 600025, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Colloidal bimetallic gold core palladium shell nanoparticles were prepared by wet chemical method, in which Au(III) and Pd(II) ions in an aqueous solution in the presence of a cationic surfactant, Cetyltrimethylammonium bromide (CTAB). The structure and composition of the metallic nanoparticles were characterized by UV-Vis, HRTEM, SEM-EDX, XRD, XPS and FTIR. The catalytic activities of nanoparticles are tested on the surface chemical characterization study of Cetyltrimethylammonium bromide supported Au-Pd bimetallic nanoparticle catalyst, hereafter named Au-Pdnp, is reported. Such a catalyst was developed for the Suzuki coupling reaction and found excellent catalytic activity. Here we describe the catalytic performance and the FTIR studies provide proof of the mode of binding that occurs in the Palladium nanoparticle surface for the first time and also confirms the mechanism of the Suzuki reaction.
Keywords: Bimetallic Nanoparticle, Suzuki Coupling Reaction, Surface Study, Thermal Method, FT-IR Study
Article Outline
1. Introduction
- Nanoparticles exhibit significantly different properties relative to those of their corresponding bulk materials and, as such, are of interest for applications in catalysis[1 - 8], magnetic[9 - 11] , drug delivery systems[12 , 13] , semiconductors[14, 15], single electron tunnelingdevices [16], nonlinear optical devices[17] , electron microscopy markers[18] and DNA sequencing[19]. Of late, bimetallic nanoparticles are of great interest because of the modification of properties observed not only due to size effects, but also as a result of the combination of different metals, either as an alloy or as a core-shell structure, modifying the catalytic properties of the monometallic nanoparticles[20 - 26]. Recently Palladium-catalyzed Suzuki cross-coupling reaction has emerged as one of the most powerful, attractive, and widely utilized method for the construction of carbon–carbon bonds[27-30]. In recent years, there has been considerable interest in the preparation of new and highly active palladium catalysts to facilitate such transformation[31-37]. Water as solvent in transition-metal catalysis has many advantages for the recycling of catalyst, product recovery and environmental aspects[38]. The beneficial effects of using water as solvent especially in Suzuki reactions are well documented[39,40]. In the present paper a surface chemical characterization study ofCetyltrimethyl ammonium bromide supported Au-Pd bimetallic nanoparticle catalyst, hereafter named Au-Pdnp, is reported. Such a catalyst was developed for the Suzuki coupling reaction and found excellent catalytic activity. Here we describe the catalytic performance and the FTIR studies provide proof of the mode of binding that occurs in the Palladium nanoparticle surface for the first time and also confirms the mechanism of the Suzuki reaction.
2. Experimental
2.1. Preparation of Au-Pd Bimetallic Nanoparticles
- The bimetallic nanoparticles were synthesized by chemical method reduction of the Au3+ and Pd2+ ions (metal salts) using sodium borohydride (NaBH4) as the reducing agent by our group previously established methods[41] The resulting colloidal nanoparticle solutions was very stable over extended periods of several months.
2.2. Catalytic Study: Suzuki Reaction between Phenyl Halide Derivatives and Phenylboronic Acid to Substituted Biphenyl: General Procedure
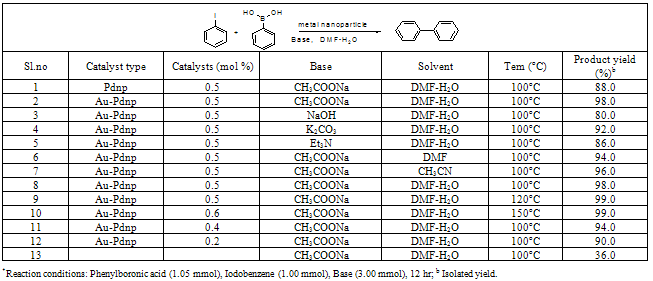
- Phenylboronic acid (1.05 mmol), Iodobenzene (1.0 mmol), base (3 mmol) and 0.5 mol % catalyst (Au-Pdnp) were introduced under argon atmosphere into a 25-ml roundbottomed, two-necked flask equipped with a stirring magnetic bar, a bubble condenser, and a silicon stopper. The reaction mixture was magnetically stirred at the required temperature (Table 1). When needed, small samples of the reaction mixture were taken from the stoppered side neck. For GC analysis the samples were treated with either water or 0.5 M aqueous HCl. The organic products were then extracted with diethyl ether, dried over anhydrous sodium sulfate and analyzed by GCMS. The coupling product was purified by column chromatography (silica, solvent: n-hexane/ethyl acetate).
2.3. FTIR Studies of Dried Films
- FTIR experiments were carried out on a Bruker Tensor 27 spectrophotometer. The spectra were collected in the range 4000 to 400 cm-1 in the transmittance mode. Solid samples were finely ground, dispersed in spectroscopic grade KBr and made into pellets. Liquid samples prepared in volatile solvents were drop cast onto the KBr pellets and allowed to dry before recording the IR spectra. A few drops of the solution were placed on a KBr pellet and allowed to dry before IR spectra were recorded.
3. Results and Discussion
3.1. Investigation of Catalytic Activity of Suzuki Reaction
- The catalytic activity of the bimetallic nanoparticles towards the Suzuki coupling reaction of Phenylboronic acid react with iodobenzene was studied, and the formation of biphenyl were observed. GC results confirmed the presence of biphenyl as a product. In the present work, the synthesis of biphenyl using a catalytic amount of bimetallicnanoparticles under DMF-water (3:1, v/v) solvents conditions. The reaction of Iodobenzene and Phenylboronic acid with Sodium acetate in the presence of catalytic amount of metal nanoparticles at 100℃ affords the desired biphenyl compounds in 99% yield (table 1, Sl.no 2). We also examined this reaction in different solvents, base, catalysts and temperature condition (Table 1). The product percentage yields obtained from different reactantcompositions show the optimum reactant compositions for maximum yield as those mentioned in the Suzuki reaction procedure (table 1). The results of product yields given in table 1 are determined by using the optimum reactant compositions only. The Sl.no 1 and 2 in the table 1 refer to the inferences obtained from the effects of catalysts nature. Among the metallic nanoparticles system, Au-Pd bimetallicnanoparticle catalysis shows maximum yield than mono metallic palladium nanoparticle.The Sl.no 2, 3 to 5 in the table 1 refer to the results of the effect of the nature of base (3.0 mmol) keeping the reaction temperature (100℃), solvent (DMF-water 3:1, v/v mixture) and 0.5 mole % catalyst as constant parameters. Maximum product yields for Au-Pd nanoparticle catalysts are found with Sodium acetate as the base and at 100℃. When Suzuki reaction was carried out with different solvents such as DMF–water and acetonitrile (table 1, Sl. no 2, 6 and 7), the product yields are found lesser for the DMF-water 3:1 mixture as the solvent. The temperature effect studies show that, the optimum reaction temperature is found at 100℃, since 120℃and 150℃ reaction temperatures also produced the same percentage yield (table 1, Sl. no. 2, 8 and 9). In all, 3.0 mmol Sodium acetate incorporated Suzuki reaction, when carried out in DMF-water (3:1) solvent mixture, loaded with 0.5 mole % of the catalyst, at 100℃ produced the maximum percentage yield of the product in the least reaction time (12 h) when the catalyst is Au-Pd bimetallic nanoparticle. The Sl. no. 2, 10 to 12 in the table 1 refer to the inferences obtained from the effects of catalysts loading to the Suzuki reaction. Among the various concentration of metallic nanoparticles system, Au-Pd nanoparticle with 0.5 and 0.6 mol % catalysis shows maximum yield compared to the other nanoparticles system. This reaction studied without catalysts (Sl. no. 13 in the table 1) gave only 36.0 % of the Suzuki products.
3.2. The Study of Reaction Mechanism FT-IR Studies on the Suzuki Reaction Mechanism as KBr Pellets
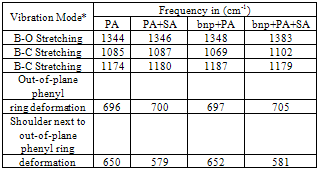
- KBr enriched films of Sodium acetate (SA),Phenylboronic acid (PA), SA + PA, CTAB-bimetallic nanoparticles, bnp + PA, and bnp + SA + PA are prepared and dried in the oven for 30 minutes in order to investigate how the Phenylboronic acid interacts with the bnp surface. In addition, films of iodobenzene (I), SA + I, and bnp + SA + I are also prepared in order to investigate how the Iodobenzene interacts with the bnp surface. FTIR spectra of the films are recorded in the range of 4000 to 400 cm-1. Table 2 summarizes the important vibrational modes and the frequencies observed in Phenylboronic acid, Phenylboronic acid + sodium acetate, Phenylboronic acid + bimetallic nanoparticles, and Phenylboronic acid + sodium acetate + bimetallic nanoparticles.
|
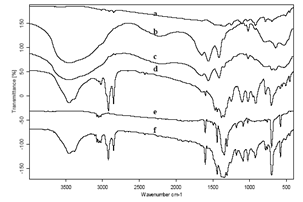 | Figure 1. (a-f) a-f FTIR spectra of Phenylboronic acid (PA), Sodium acetate (SA), SA + PA, bimetallic nanoparticles (bnp), bnp + PA, and bnp + SA + PA in 4000 to 400 cm-1 region (a) bnp, (b) SA, (c) PA, (d) SA + PA, (e), PA + bnp, (f) bnp + SA + PA at 25℃ |
 | Figure 2. Illustration of phenylboronate anion and the two possibilities of binding to the Au-Pd nanoparticle surface which can occur. The binding can occur through one B-O- group or through both B-O- groups |
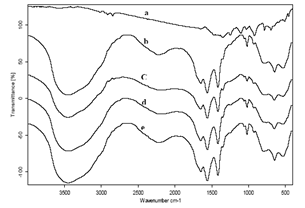 | Figure 3. a-e FTIR spectra of Iodobenzene (I), Sodium acetate (SA), bimetallic nanoparticles (bnp), SA + I, and bnp + SA + I in 4000 to 400 cm-1 region (a) bnp, (b) SA, (c) I, (d) I + SA, (e) I + SA + bnp at 25℃ |
4. Conclusions
- In conclusion, we have synthesized a novel family of Au-Pd core-shell bimetallic nanoparticles from the wet chemical method of metal-surfactant complexes has been reported our previously report. We have demonstrated that a colloidal solution of Au-Pd bimetallic nanoparticles is an efficient catalyst for Suzuki cross-coupling reactions in aqueous solution. The surface of palladium has a reasonably high surface area and pore volume making it an attractive host for the insertion of nanoparticles. The size of the bimetallic nanoparticle could be controlled within the CTAB. The UV-Vis, HRTEM, XRD, SEM-EDX, XPS and FTIR analyses all suggest the formation of a core-shell Au-Pd structure. The catalytic activity of the Suzuki couplingreaction has monitored by FTIR spectroscopy. The bimetallic nanoparticles were found to have high catalytic activity than mono metallic nanoparticle. The high catalytic activity of bimetallic nanoparticles is probably due to the sequential electronic effect between elements in a particle. More detailed investigations of nanoparticle structure effects on the catalytic activity and their applicability in other synthetic transformations are currently under investigation.
ACKNOWLEDGMENTS
- The authors thank financial support to the Department from DST-FIST
References
| [1] | Alain Roucoux, Jurgen Schulz, and Henri Patin, Chem. Rev. 102 (2002) 3757 - 3778. |
| [2] | Peiwen Zheng, and Wangging Zhang. J. catal. 250 (2007) 324 - 330. |
| [3] | L. N. Lewis, Chem. Rev. 93 (1993) 2693 - 2730. |
| [4] | J. D. Aiken, Y. Lin, and R. G. Finke, J. Mol. Catal. A: Chem. 114 (1996) 29 - 51. |
| [5] | H. Tsunoyama, H. Sakurai, Y. Negishi, and T. Tsukuda, J. Am. Chem. Soc. 127 (2005) 9374 - 9375. |
| [6] | Yin Li, Edna Boone, and Mostafa A. El-Sayed, Langmuir. 18 (2002) 4921 - 4925. |
| [7] | J. Dai, and M. L. Bruening, Nano Lett. 2 (2002) 497 - 501. |
| [8] | W. Yoo, D. Hathcock, and M. A. El-Sayed, J. Phys. Chem. A. 106 (2002) 2049 - 2054. |
| [9] | M. Nakaya, M. Kanehara, and T. Teranishi, Langmuir. 22 (2006) 3485 - 3487. |
| [10] | X. Du, and N. Toshima, Chem. Lett. 35 (2006) 1254 - 1255. |
| [11] | M. Chen, J. Kim, J. P. Liu, H. Fan, and S. Sun, J. Am. Chem. Soc. 128 (2006) 7132 - 7133. |
| [12] | S. O. Keun, E. L. Kyung, S. H. Sung, H. C. Sun, K. Dongmin, and Y. Soon Hong, Biomacromolecules. 6 (2005) 1062 - 1067. |
| [13] | Ajay Kumar Gupta, Stephen Wells, and Ieee Transactions On Nanobioscience, 3 (2004) 66 - 84. |
| [14] | T. Teranishi, M. Nishida, and M. Kanehara, Chem. Lett. 34 (2005) 1004 - 1005. |
| [15] | N. Watanabe, and N. Toshima, Bull. Chem. Soc. Jpn. 80 (2007) 208 - 214. |
| [16] | R. P. Andres, T. Bein, M. Dorogi, S. Feng, J. J. Henderson, C. P. Kubiak, W. Mahoney, R. G. Osifchin, and R. Reifenberger. Science 272 (1996) 1323 - 1325. |
| [17] | P. Galletto, P. F. Brevet, H. H. Girault, R. Antoine, and M. Broyer. J. Phys. Chem. B 103 (1999) 8706 - 8710. |
| [18] | W. Baschong, and N. G. Wrigley, J. Electron Microsc. Tech. 14 (1990) 313 - 323. |
| [19] | W. J. Parak, T. Pellegrino, C. M. Micheel, D. Gerion, S. C. Williams, and A. P. Alivisatos, Nano Lett. 3 (2003) 33 - 36. |
| [20] | Shiho Tokonami, Nobuyasu Morita, Kanako Takasaki, and Naoki Toshima J. Phys. Chem. C. (2010, in press) |
| [21] | Karl J. J. Mayrhofer, Katrin Hartl, Viktorija Juhart, and Matthias Arenz J. Am. Chem. Soc., 2009, 131 (45), 16348–16349. |
| [22] | N. Aihara, K. Torigoe, and K. Esumi, Langmuir 14 (1998) 4945 - 4949. |
| [23] | P. Laura, V. Alberto, C. Claudio, and S. Paolo, Topics in Catal. 44 (2007) 319 - 335. |
| [24] | J.K. Edwards, B.E. Selsone, P. Landon, A.F. Carley, A. Herzing, C.J. Kiely, and G.J. Hutchings, J. Catal. 236 (2005) 69 - 79. |
| [25] | M.O. Nutt, J.B. Hughes, and M.S. Wong, Environ, Sci. Technol. 39 (2005) 1346 - 1353. |
| [26] | Maureen R. Regan, and Ipsita A. Banerjee, Scripta Materialia. 54 (2006) 909 - 914. |
| [27] | N. Miyaura, T. Yanagi, and A. Suzuki, Synth. Commun. 11 (1981) 513 - 519. |
| [28] | N. Miyaura, and A. Suzuki, Chem. Rev. 95 (1995) 2457 - 2483. |
| [29] | A. Suzuki, J. Organomet. Chem. 576 (1999) 147 - 148. |
| [30] | N. Miyaura, Top. Curr. Chem. 219 (2002) 11 - 59. |
| [31] | N. Kataoka, Q. Shelby, J.P. Stambuli, and J.F. Hartwig, J. Org. Chem. 67 (2002) 5553 - 5566. |
| [32] | M. Miura, Angew. Chem., Int. Ed. 43 (2004) 2201 - 2203. |
| [33] | K.W. Anderson, and S.L. Buchwald, Angew. Chem., Int. Ed. 44 (2005) 6173 - 6177. |
| [34] | K. Sarkar,M. Nandi, M. Islam,M.Mubarak, and A. Bhaumik, Appl. Catal. A: Gen. 352 (2009) 81 - 86. |
| [35] | M. Joshaghani, E. Faramarzi, E. Rafiee, M. Daryanavard, J. Xiao, and C. Baillie, J. Mol. Catal. A: Chem. 259 (2006) 35 - 40. |
| [36] | T.E. Barder, J. Am. Chem. Soc. 128 (2006) 898 - 904. |
| [37] | P. Han, H.M. Zhang, X.P. Qiu, X.L. Ji, and L.X. Gao, J. Mol. Catal. A: Chem. 295 (2008) 57 - 67. |
| [38] | C. J. Li, Chem. Rev. 105 (2005) 3095 - 3166. |
| [39] | E. Alacid, and C. Najera, Org. Lett. 10 (2008) 5011 - 5014. |
| [40] | . Ma, X. L. Cui, L. Y. Gao, and Y. J. Wu, Inorg. Chem. Commun. 10 (2007) 762 - 766. |
| [41] | J. Santhanalakshmi, and P. Venkatesan. J. nanopart. Res. 13, (2011), 479 – 490 |
| [42] | J. A. Creighton, and D. G. Eadon, J. Chem. Soc., Faraday Trans. 87 (1991) 3881 - 3891. |
| [43] | N. Toshima, and T. Yonezawa, New J. Chem. 22 (1998) 1179 - 1201. |
| [44] | N. Toshima, M. Harada, Y. Yamazaki, and K. Asakura, J. Phys. Chem. 96 (1992) 9927 - 9933. |
| [45] | J. A. Creighton, and D. G Eadon, J. Chem. Soc. Faraday Trans., 87 (1991) 3881 - 3891. |
| [46] | T. E. Furtak, and J. Kester, Phys. Rev. Lett. 45 (1980) 1652 - 1655. |
| [47] | C. J. Mathews, P. J. Smith, and T. Welton, Chem. Commun. (2000) 1249 - 1250. |
| [48] | R. Rajagopal, D. V. Jarikote, and K. V. Srinivasan, Chem. Commun. (2002) 616 - 617. |
| [49] | J. D. Revell, and A. Ganesan. Org. Lett. 4 (2002) 3071 - 3073. |
| [50] | R. Narayanan, R. J. Lipert, and M. D. Porter, Anal. Chem. 80 (2008) 2265 - 2271. |
| [51] | D. Syomin, and B. E. Koel. Surf. Sci., 490 (2001) 265 - 273. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML