-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2012; 2(3): 66-71
doi: 10.5923/j.materials.20120203.06
Evolution of Structure, Microstructure, Electrical and Magnetic Properties of Nickel Oxide (NiO) with Transition Metal ion Doping
1Department of Physics, North Orissa University, Baripada 757003, India
2Department of Physics, Utkal University, Bhubaneswar 751004, India
Correspondence to: P. Mallick, Department of Physics, North Orissa University, Baripada 757003, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
We present a brief review on the evolution of structure, microstructure, electrical and magnetic properties of NiO with transition metal (TM) doping. The fcc structure of NiO is not affected with TM doping whereas the some of the TM ion influences the microstructure. The antiferromagnetic property of NiO is strongly modified with some of the TM (Fe, Mn, V) doping and the same is not much affected with some other TM (Co, Cr, Zn and Cu) doping. Not only the dopants but also the form of the material decides the magnetic order in the host matrix. Powder NiO exhibits room temperature ferromagnetism with Fe doping, superparamagnetism with Mn doping. NiO thin films on the other hand exhibit ferromagnetism with Fe, Mn and V doping. The ferromagnetic ordering in these cases was improved with Li co-doping. The increased ferromagnetism in these cases may be due to increase of hole concentration due to Li doping. Giant dielectric response has been observed for (Li, Fe) and (Li, V) doped NiO ceramics.
Keywords: Diluted Magnetic Semiconductors; Doping; Transition Metal Oxide; Nio
Article Outline
1. Introduction
- The different properties that a material exhibits can undergo extensive modification on doping with foreign elements. There are situations where the host matrix only acts as a vehicle, but its property is solely dictated by the dopants. The induction of extrinsic conductivity (either p or n type) in silicon, germanium semiconductors on trivalent or pentavalent ion doping, emergence of high TC superconductivity or colossal magneto-resistivity on doping of cuprateperovskites and manganites, tuning of band gap in materials for applications in solar cell technology are a few out of a large varieties of phenomena and applications that emerge on doping. Though many elements in the periodic table can be doped in a host matrix depending upon the chemical compatibility, the transition metal elements have specific advantages when used as dopant. NiO has been considered as a promising functional material for varieties of applications as smart windows, spin valves giant magneto resistance (GMR) sensor, solar cells,p-type transparent conducting electrodes, gas sensors, high permittivity dielectric material and electrochromic material for displays etc. TM doped NiO samples have been studied mainly for their dielectric and magnetic properties. However, the major focus has been confined to the evolution of magnetic property in NiO with TM doping.High-permittivity dielectric materials with good thermal stability have been played a significant role in microelectronics. Giant dielectric response has been observed for (Li, Fe) and (Li, V) doped NiO[1,2]. The effects of transition metal like Fe, Co, Mn etc. doping can drive a non-magnetic semiconductor into a semiconductor with ferromagnetism. This has led to the emergence of a new field of research, the diluted magnetic semiconductors (DMS). DMS has charge and spin degrees of freedom in a single substance to realize a new class of spintronic devices. The potential advantages of spintronic devices will be higher speed, greater efficiency, and better stability, in addition to the low energy required to flip a spin[3]. For practical applications, the DMS materials need to exhibit high Curie temperature (>300 K) with intrinsic ferromagnetism rather than one due to the presence of secondary phases.It is anticipated that if one can introduce room temperature ferromagnetism in cubic systems, it will facilitate the integration of spintronic devices with advanced silicon based microelectronic devices[4]. Dietlet al.[5] had predicted that the curie temperature of p-type DMS can be well above that of n-type DMS. NiO exhibits fcc structure. It is a prototype p-type transparent conducting oxide[6,7] due to vacancy at Ni2+ sites[8,9]. NiO as such is a transition metal oxide. Doping of other transition metal elements at Ni site modifies the magnetic, electrical, optical and to some extent structural properties, and opens up the possibility of exploiting this material for DMS functionality. Here, we present a brief review on the evolution of structure, microstructure, electrical and magnetic properties of NiO, on doping with different TM elements.
2. Evolution of Structure and Microstructure of Nio with Transition Metal Doping
- Bulk NiO exhibits cubic (fcc) structure with a slight rhombohedral distortion below the Neel temperature, TN (~523 K).This distortion is believed to be a consequence of the confinement of ferromagnetically ordered spins within the (111) plane[10]. Due to this spin structure, the exchange coupling to the nearest neighbours within the ferromagnetic planes is slightly larger than that to the nearest neighbours out of these planes, with antiferromagntic coupling between adjacent planes. This exchange striction manifests itself in NiO not being perfectly cubic but having a small contraction of the distance between the ferromagnetically ordered planes below TN[11]. However, the difference in plane spacing is only about 0.1% at 300 K[11] and this deviation from cubic symmetry is in general ignored[12]. The fcc structure of NiO is retained with different TM doping such as Fe, Co, Mn by different concentrations within the solubility limit[13-16]. However the microstructure of the host NiO matrix is influenced considerably by the dopant ions.In general TM monoxides exhibit either cation or anion vacancies. The former category includes ZnO, CdO, VO, etc., while the latter category includes NiO, FeO, CoO and MnO. In the latter category of transition metal monoxide (TMO), the formation of microstructural defects, such as metal vacancies and interstitial oxygen has been seen. Among these TMOs, NiO has been shown to exhibit least amount of non-stoichiometry in its bunsenite form, Ni1-δO (δ<1
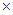 10-3)[17]. The values of δ for Fe1-δO, Mn1-δO and Co1-δO are 0.05-0.15, 0.001-0.15 and 0.001-0.05 respectively[17]. Due to the difference in non-stoichiometry, any of the ions like Fe, Co, Mn if used as dopant, is expected to govern the defect structure of NiO. The Fe1-δO in its wustite state is well known to produce the defect clusters, consisting of cation vacancies and iron interstitials[18-20]. The basic unit of these clusters, the so-called (4:1)-clusters, consists of four cation vacancies, tetrahedrally coordinating a trivalently charged iron interstitial ion. This gives rise to the generation of 5 holes around the defect structure. As in wustite, the 4:1 clusters can occur in the bunseniteNiO due to Fe doping. In this case a Fe3+ ion occupying interstitial site is coordinated to 4 Ni vacancies. Such a defect structure in Fe doped NiO was predicated by theoretical studies[17] and indicated by neutron scattering experiments[21]. Similar is the situation with Mn and Co doping, where the defect structure in MnO and CoO is expected to be reflected in the structure of NiO if doped by Mn or Co. The available literature on TM doped NiO indicates that only a few studies have been undertaken on the bulk samples[22-24]. Most of the studies on TM doped NiO are confined to nanostructure rather than bulk. In some cases, though attempts have been made to synthesize bulk phase samples, the segregation of secondary phases prevails and inclusion of nanoparticles of dopants in the host matrix occurs.The particle size of NiO increases on increasing annealing temperature[25,26] and annealing time[25]. Interestingly, the particle size of NiO has been shown to decrease with increasing TM doping concentration[13,15,27]. Similar type of observation has been reported for Mn doped (ZnAs)O also, where Mn was shown to be a potential catalyst for nano dot formation[28]. The exact role of TM ions in reducing the particle size is still elusive.Li is not a transition metal. However Li doping has been shown to influence the non-stoichiometry of NiO. Due to its valence state being different from that of Ni, its co-doping with another TM ion into NiO is expected to affect the defect structure as well as the physical properties of NiO. The Ni0.98-xFe0.02LixO samples show improved texturing along <111> with increasing Li concentration[29]. The texture evolution of NiO has been shown to be governed by crystallinity and grain size[30]. Increasing substrate temperature during the film growth by RF sputtering results into increased grain size and leads to suppression of texture along <111> and the evolution of the same along <200>. Therefore the enhanced texture along <111> indicates that Li doping suppresses the crystallinity and hence grain size. Like (Li, Fe) doped NiO case, 1 wt% Co doped NiO also showed the texturing along <111>[31], which may be due to the suppression of grain size. On the contrary, the grain size was found to remain unaffected with Co concentration in Co, Li co-doped NiO[32]. The grain size also remains unaffected in low doping concentration of Mn in NiO and the same decreased at higher Mn concentration[16].
10-3)[17]. The values of δ for Fe1-δO, Mn1-δO and Co1-δO are 0.05-0.15, 0.001-0.15 and 0.001-0.05 respectively[17]. Due to the difference in non-stoichiometry, any of the ions like Fe, Co, Mn if used as dopant, is expected to govern the defect structure of NiO. The Fe1-δO in its wustite state is well known to produce the defect clusters, consisting of cation vacancies and iron interstitials[18-20]. The basic unit of these clusters, the so-called (4:1)-clusters, consists of four cation vacancies, tetrahedrally coordinating a trivalently charged iron interstitial ion. This gives rise to the generation of 5 holes around the defect structure. As in wustite, the 4:1 clusters can occur in the bunseniteNiO due to Fe doping. In this case a Fe3+ ion occupying interstitial site is coordinated to 4 Ni vacancies. Such a defect structure in Fe doped NiO was predicated by theoretical studies[17] and indicated by neutron scattering experiments[21]. Similar is the situation with Mn and Co doping, where the defect structure in MnO and CoO is expected to be reflected in the structure of NiO if doped by Mn or Co. The available literature on TM doped NiO indicates that only a few studies have been undertaken on the bulk samples[22-24]. Most of the studies on TM doped NiO are confined to nanostructure rather than bulk. In some cases, though attempts have been made to synthesize bulk phase samples, the segregation of secondary phases prevails and inclusion of nanoparticles of dopants in the host matrix occurs.The particle size of NiO increases on increasing annealing temperature[25,26] and annealing time[25]. Interestingly, the particle size of NiO has been shown to decrease with increasing TM doping concentration[13,15,27]. Similar type of observation has been reported for Mn doped (ZnAs)O also, where Mn was shown to be a potential catalyst for nano dot formation[28]. The exact role of TM ions in reducing the particle size is still elusive.Li is not a transition metal. However Li doping has been shown to influence the non-stoichiometry of NiO. Due to its valence state being different from that of Ni, its co-doping with another TM ion into NiO is expected to affect the defect structure as well as the physical properties of NiO. The Ni0.98-xFe0.02LixO samples show improved texturing along <111> with increasing Li concentration[29]. The texture evolution of NiO has been shown to be governed by crystallinity and grain size[30]. Increasing substrate temperature during the film growth by RF sputtering results into increased grain size and leads to suppression of texture along <111> and the evolution of the same along <200>. Therefore the enhanced texture along <111> indicates that Li doping suppresses the crystallinity and hence grain size. Like (Li, Fe) doped NiO case, 1 wt% Co doped NiO also showed the texturing along <111>[31], which may be due to the suppression of grain size. On the contrary, the grain size was found to remain unaffected with Co concentration in Co, Li co-doped NiO[32]. The grain size also remains unaffected in low doping concentration of Mn in NiO and the same decreased at higher Mn concentration[16].3. Evolution of Electrical Properties of NiO with Transition metal Doping
- The electrical conductivity of many metal oxide semiconductors is extensively influenced by impurity doping. The classical example is the dramatic increase in electrical conductivity of silicon and germanium with parts per million (ppm) level of impurity. As such NiO is a hole doped p-type semiconductor. The electrical conductivity of undopedNiO has a strong dependence on the formation of microstructural defects, such as nickel vacancies and interstitial oxygen in NiO crystallites[33]. With proper thermal treatment, it becomes slightly non-stoichiometric, acquiring an excess of oxygen, which is compensated by the oxidation of some Ni2+ to Ni3+. In the ground state, the extra charge of Ni3+ is trapped close to the Ni vacancy[34,35] and can move only by an activated hopping process similar to that exhibited by ionic diffusion. Therefore, this kind of compound is generally designated as a hopping semiconductor[36]. The non-stoichiometry and hence p-type conductivity in NiO can be further enhanced by doping of aliovalent metal ions like Li at Ni site[13,37]. Intensive investigations have been conducted in doped NiO for its great theoretical and technological importance due to the special electrical behaviours and related spin dynamics[9,38].NiO has also been shown to evolve with different properties like high dielectric permittivity[1,2,39,40], thermoelectric behaviour[41,52], ferromagnetism[13,24-27,29,43] etc. on doping. However only a few studies have been undertaken on the electrical properties of TM doped NiO.The activation energy and hence the frequency dependent electrical conductivity of NiO have been shown to sensitively depend on Fe doping at very low concentrations. Fe doping (0.5 at.%) leads to increase of the activation energy of NiO (from ~0.51eV to ~0.85 eV). Further increasing Fe concentration has a little influence on the activation energy, which probably is due to the solubility restrictions[27]. Like Fe doping case, the activation energy for the nanocrystallineMn doped NiO was found to be greater than that of undopedNiO[44]. Co-doping of NiO with TM ions and Li+ ions have been attempted to induce changes in both magnetic and electrical properties, which can induce DMS behaviour as discussed later. As such the replacement of Ni2+ by Li+ increases hole carrier concentration[45,46]. The occurrence of higher conductivity has been reported for Ni0.98-xFe0.02LixO samples with increasing Li concentration[29]. The increased conductivity with Li concentration may be due to the increase of the hole concentration and the decrease of the activation energy, as observed in the LixNi1−xO system[46].NiO in recent years has been considered as an important dielectric material due to its high-permittivity when doped with different elements. Among the different element doped NiO, (Li, Fe) and (Li, V) doped NiO have shown to exhibit giant dielectric response[1,2]. The observation of high dielectric constant in doped NiO is attributed mainly to a grain boundary barrier layer capacitance[9,47,48], which can arise due to the formation of core/shell structure with conductive grain as core and resistive boundary as shell[1,2].
4. Evolution of Magnetic Properties of Nio with Transition Metal Doping
- NiO in its bulk form shows antiferromagnetic behaviour. The magnetic structure of NiO consists of ferromagnetic sheets of Ni2+ parallel to the (111) plane with opposite spin directions in neighboring sheets. Magnetic properties of NiO, in addition to depending on TM ion doping, also sensitively depends on the size of the particles in nanoscale of the particle size. We thus present a brief discussion on particle size effect before going over to TM doping effect on magnetic properties of NiO.When the dimension is reduced to nanoscale, the antiferromagnetic material like NiO is shown to exhibit different magnetic properties like ferromagnetism[49] (for particles of size,
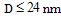 ), superparamagnetism (
), superparamagnetism (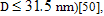 spin glass behavior (D
spin glass behavior (D 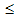 10 nm)[51,52] and even core-shell like structure (4
10 nm)[51,52] and even core-shell like structure (4 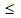 D
D 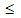 22 nm)[53], where the core of NiO nanoparticle behaves like a ferrimagnet and the shell contains randomly oriented spins with low co-ordination. Thus depending upon the particle size, different anomalous magnetic properties emerge at the expense of antiferromagnetic property of the host NiO. NiO nanoparticles are shown to exhibit finite size effect, where 8-, 6-, or 4-sublattice spin configurations arise due to the reduced coordination of surface spins leading to anomalous magnetic properties like large moments and coercivity, and loop shifts[50]. These anomalous properties however emerge only when the particle size is less than a critical size (31.5nm)[50].The effect of TM iondoping on the magnetic properties of NiO is still not explored well. In recent years, TM ions such as Fe, Co, Mn etc. have been used as dopant for NiO. Fe doping in NiO leads to ferromagnetism in both nanoparticle and thin film form whereas Mn and V doped NiO exhibits ferromagnetism in thin film form. The TM iondoped materials which exhibit ferromagnetism is generally regarded as Diluted magnetic semiconductors (DMS) and are important class of materials due to their potential applications for spintronic devices[54,55]. The studies on the evolution of ferromagnetism in TM doped NiO are mostly confined either to nanoparticle powders or to thin films. Attempts have been made to synthesize bulk Fe doped NiO either by solid state reaction method[24] or by high temperature annealing of nanoparticles[25,26]. These studies however indicated phase segregation and precipitation of secondary impurity phase of doped ions, questioning the intrinsic nature of DMS. On the contrary, the Co doped NiO did not show any impurity phase. It also did not show any drastic change in magnetic property unlike Fe doping case. Though Li is not a TM ion, its doping into NiO has been shown to considerably influence the electrical as well as the magnetic properties[56-58]. We therefore discuss literatures on the evolution of magnetic properties on TM doping as well as on (Li, TM) doping in NiO.
22 nm)[53], where the core of NiO nanoparticle behaves like a ferrimagnet and the shell contains randomly oriented spins with low co-ordination. Thus depending upon the particle size, different anomalous magnetic properties emerge at the expense of antiferromagnetic property of the host NiO. NiO nanoparticles are shown to exhibit finite size effect, where 8-, 6-, or 4-sublattice spin configurations arise due to the reduced coordination of surface spins leading to anomalous magnetic properties like large moments and coercivity, and loop shifts[50]. These anomalous properties however emerge only when the particle size is less than a critical size (31.5nm)[50].The effect of TM iondoping on the magnetic properties of NiO is still not explored well. In recent years, TM ions such as Fe, Co, Mn etc. have been used as dopant for NiO. Fe doping in NiO leads to ferromagnetism in both nanoparticle and thin film form whereas Mn and V doped NiO exhibits ferromagnetism in thin film form. The TM iondoped materials which exhibit ferromagnetism is generally regarded as Diluted magnetic semiconductors (DMS) and are important class of materials due to their potential applications for spintronic devices[54,55]. The studies on the evolution of ferromagnetism in TM doped NiO are mostly confined either to nanoparticle powders or to thin films. Attempts have been made to synthesize bulk Fe doped NiO either by solid state reaction method[24] or by high temperature annealing of nanoparticles[25,26]. These studies however indicated phase segregation and precipitation of secondary impurity phase of doped ions, questioning the intrinsic nature of DMS. On the contrary, the Co doped NiO did not show any impurity phase. It also did not show any drastic change in magnetic property unlike Fe doping case. Though Li is not a TM ion, its doping into NiO has been shown to considerably influence the electrical as well as the magnetic properties[56-58]. We therefore discuss literatures on the evolution of magnetic properties on TM doping as well as on (Li, TM) doping in NiO.4.1. Fe and (Li, Fe) Doped NiO
- Ferromagnetism has been shown to evolve in NiO at room temperature on doping with 2 at.% Fe[13,15,26,27,43]. However the origin of the occurrence of ferromagnetism is still not clear. In order to get information about the origin of ferromagnetism in Fe doped NiO,Douvalis et al.[25] prepared Ni0.98Fe0.02O by chemical method and heated this precursor in air at temperatures between 673 and 873 K, for 1 to 25 h. Their magnetization measurements show that samples heated both at 673 and 873 K exhibit room-temperature ferromagnetism. However their Mossbauer spectroscopy results indicate that this ferromagnetism is related to Ni57Fe2O4ferrimagnetic impurities for the samples heated at 873 K and to finite-size effects for the samples heated at 673 K. This observation thus indicated that ferromagnetism in Fe doped NiO is not due to Fe induced DMS effect as was initially presumed by many[13,27], but due to second phase precipitation. Thermogravimetric and magnetic study of polycrystalline Fe doped NiO samples prepared by solid state reaction method by Raja et al.[24] also indicated that the observed ferromagnetism in these samples is due to second phase (NiFe2O4) precipitation like that observed by Douvalis et al.[25].Li doping into NiO leads to the generation hole as charge carriers. Li co-doping was introduced into the TM doped NiO with an aim to achieve strong carrier mediated ferromagnetism at room temperature. The evolution of magnetic ordering in the Fe, Li co-doped NiO showed an opposite behaviour in polycrystalline powder samples than in thin films with increasing Li concentration. The powder Ni0.98-xFe0.02LixO samples[37] did not show the enhanced ferromagnetic properties unlike the Ni0.98-xFe0.02LixO thin films[29,59].In polycrystalline Fe, Li co-doped NiO powder samples, the ferromagnetic transition temperature was shown to decrease with increasing Li concentration. In this case, the random substitution of Li in Fe doped NiO leads to the formation of ferromagnetic phases locally which is embedded inside the antiferromagnetic host NiO. The coexistence of ferromagnetic and antiferromagnetic phases lead to spin glass type behavior in NiO system as a result of which ferromagnetic transition temperature decreased[37].In case of Fe, Li co-doped NiO thin films, the room temperature ferromagnetic ordering was shown to improve with increasing Li concentration[59]. Since holes are generated on Li doping in NiO, the density of holes increases with increasing Li concentration. More hole carriers are expected to enhance the ferromagnetic coupling interaction between the hole carriers and the Fe 3d spins, thus making ferromagnetic coupling interaction more effective as was reported for Fe, Li co-doped thin films[29,59]. Similar phenomena have also been observed in the Li, Co co-doped ZnO and Cu, Fe co-doped ZnO thin films[60,61].
4.2. Co and (Li, Co) Doped NiO
- The magnetic structure of the solid solutions ofNi1-xCoxO as a function of composition and temperature has been studied by neutron diffraction technique[23]. As indicated from this study, the antiferromagnetic ordering persists in Co doped NiO. The TN decreased linearly with increasing Co concentration in NiO[62]. Since the TN of CoO and NiO are ~ 287 K and 523 K respectively, the replacement of Co with Ni thus results into the linear decrease of TN with increasing Co concentration in Co doped NiO as observed.Li co-doping with Co inNiO thin films indicated ferromagnetic (FM) like ordering occurring at low temperature due to hole mediation as discussed earlier. Co and Li co-doped NiO nanostructured films grown of MgO (100) substrate for example exhibit FM like ordering at low temperatures (5 K), while the system is slightly paramagnetic at room temperature[32]. Antiferromagntism still dominates at room temperature. To delineate the effect of magnetic ion from the hole concentration, these authors studied the evolution of magnetization with Co concentration keeping the Li concentration fixed. The samples did not show any dramatic change in the magnetic behaviour with different Co concentration. Since the density of hole is not expected to vary with different Co concentration, it did not influence the antiferromagnetic structure of NiO much. The FM-like ordering as seen at low-temperature has been attributed to the manifestation of localized nature of the Li induced carrier and the low free carrier density[32].
4.3. Mn and (Li, Mn) Doped NiO
- The evolution of magnetization in Mn doped NiO showed different behaviour in polycrystalline power samples than in thin film from. With increasing Mn doping concentration polycrystalline NiO powder shows superparamegntism at the expense of antiferromagnetism. The antiferro to superparamagnetic transition on Mn doping in NiO is explained on the basis of Mn occupying Ni site and breaking the translational symmetry of the parent antiferromagnetic correlation[16].Mn doped NiO thin films are shown to exhibit room temperature ferromagnetic behaviour. The ferromagnetic properties of the Mn doped NiO is also shown to increase by Li co-doping like Fe doping case. The occurrence of ferromagnetism in Mn doped NiO is ascribed to intrinsic in nature. The enhancement of ferromagnetism in (Li, Mn) doped NiO is due to double exchange coupling effects via Li-induced holes[63].
4.4. Other TM (V, Cr, Cu, Zn) Doped NiO
- Recently, the effect of V, Cr, Cu and Zn doping and their co-doping with Li on the magnetic properties of NiO thin films have been studied[63]. Magnetization measurements in these cases reveal that V doped NiO thin films show room-temperature ferromagnetic behaviours and the same is shown to increase with Li co-doping like Fe and Mn doped NiO thin films. NiO thin films show antiferromagnetic behaviour with nearly zeromagnetic moments for Cr, Zn, and Cu doping. The antiferromagnetic behaviour of these samples also does not change much with Li co-doping even up to 15% of the Li concentration[63].
5. Conclusions
- The evolution of different properties of NiO on TM doping is reviewed. The structure of NiO is not being affected with TM doping whereas the microstructure is influenced by some of the TM ions. Giant dielectric response has been observed for (Li, Fe) and (Li, V) doped NiOceramics. Powder NiO exhibits room temperature ferromagnetism with Fe doping, superparamagnetism with Mn doping. Antiferromagnetic property of the host NiO either in powder form or in thin film form is not influenced with Co doping. NiO thin films on the other hand exhibit ferromagnetism with Fe, Mn and V doping. The ferromagnetic ordering in these cases improve with Li co-doping. The increased ferromagnetism in these cases may be due to increase of hole concentration due to Li doping.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML