-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2012; 2(3): 62-65
doi: 10.5923/j.materials.20120203.05
Artificial Neural Network Modeling for Al-Zn-Sn Sacrificial Anode protection of Low Carbon Steel in Saline Media
O. Oluwole, N. Idusuyi
Department of Mechanical Engineering, University of Ibadan, Nigeria
Correspondence to: O. Oluwole, Department of Mechanical Engineering, University of Ibadan, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
This work presents the artificial neural network(ANN) modeling for sacrificial anode cathodic protection of low carbon steel using Al-Zn-Sn alloys anodes in saline media. Corrosion experiments were used to obtain data for developing a neural network model. The Feed forward Levenberg-Marquadt training algorithm with passive time, pH, conductivity,% metallic composition used in the input layer and the corrosion potential measured against a silver/silver chloride(Ag/AgCl) reference electrode used as the target or output variable. The modeling results obtained show that the network with 4 neurons in the input layer, 10 neurons in the hidden layer and 1 neuron in the output layer had a high correlation coefficient (R-value) of 0.850602 for the test data, and a low mean square error (MSE) of 0.0261294. 9
Keywords: Cathodic Protection, Sacrificial Anodes, Artificial Neural Networks
Article Outline
1. Introduction
- Corrosion is the destruction of material resulting from exposure and interaction with the environment[1]. While common usage typically associates corrosion with metals, the destruction of non-metallic materials as a result of exposure to sunlight can be considered as corrosion[1]. The economic cost of corrosion was estimated some years ago by the US Department of Commerce to be approximately 5% of the Gross National Product[2]. However corrosion not only has an economic cost it can present a threat to life through the collapse of a structure or to the environment through the leak of toxic chemicals[3]. To control corrosion effectively, the accurate modeling and prediction of corrosion behaviour is a fundamental requirement[4]. Artificial neural networks are computational systems that simulate the Neurons or simple processors of a biological nervous system and have been used to solve complex engineering problems like corrosion [4,5]. Basically, all ANNs have a similar topological structure or architecture[5]. The interaction of the neurons in the network is roughly based on the principles of neural science. The neural network can learn both static and dynamic properties autonomously based on the past history of the measurement data and then act in such a way that a better solution can be obtained under unknown environmental conditions. Hence they are suitable for problems where pattern recognition is important and precise computational answers are not required[4,5].Different researchers have attempted at various times to deploy the neural network technique for the prediction of corrosion rate, which have yielded very good prediction results compared to mechanistic models[4,5,8].Artificial neural networks have also found increasing usage along side mechanistic approaches in determining evolution of corrosion rate and yield strength in certain Mg-Rare Earth alloys[13] and modeling pit growths in high strength aluminum alloys[14].However, some of these applications are advanced modeling techniques that are not readily available to the corrosion engineer who may be interested in creating his/her own neural network model for the corrosion problem at hand. Hence this paper seeks to demonstrate an attempt to create a successful neural network model for an Al-Zn-Sn sacrificial anode protection system using a simple and straightforward modeling strategy.
2. Materials and Method
2.1. Material
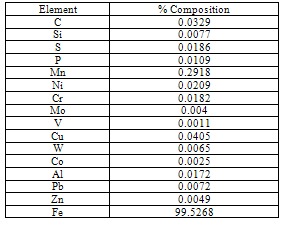
- The chemical composition of the low carbon steel sample used is presented in Table 1. The cast aluminum anodes used were Al-Zn(5%)-Sn(0.1%), Al-Zn(7%)-Sn(0.1%) and Al- Zn(10%)-Sn(0.1%).In this work, the Levenberg-Marquadt training algorithm was used to develop a network for predicting the corrosion potential of low carbon steel in saline media. Corrosion experiments were mounted to obtain the necessary data for training the networks.
2.2. Methods
2.2.1. Preparation of Samples
- The low carbon steel specimen with dimensions of 50mm x 50mm x 1mm (eight pieces) were neatly cut and polished in a sequence of emery papers until a smooth finish was obtained. They were cleaned by rinsing under running tap water, distilled water and acetone. This was done a number of times to ensure that there were no traces of corrosion products on the metal surface
|
2.2.2. Experimental Data
- Electrochemical measurements were carried out on the low carbon steel specimens with 25 cm2 exposed geometric surface, protected with the aluminum alloy anodes immersed in 1M and 0.5M NaCl solutions. The potentials were measured against an Ag/AgCl saturated reference electrode using a DT9205A digital multimeter. All measurements were carried out at a temperature of 298 K. The pH and conductivity were measured. The experiment run lasted for 630 hours.
2.3. Neural Network Application for the Sacrificial Anode Protection System
- The MATLAB ® neural network toolbox was used for the ANN modeling. The system was used to assess the potential difference required to control sacrificial anode cathodic protection systems and to predict corrosion rate. A network with two hidden layers was used. This number is chosen randomly as the selection of the number of neurons was specified by trial and error. For the potential difference assessment, the input layer received the variables (time of exposure, pH, conductivity, metal composition of the anode). The output layer gave the potential difference as network result.The tan-sigmoid transformation function was used in the first hidden and in the second layer, a pure line function. The Levenberg Marquadt feed forward neural network training algorithm was used with 107 samples.
3. Results and Discussion
3.1. Results
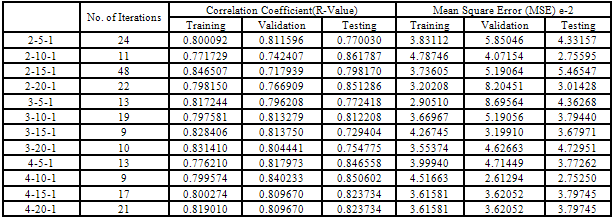
- Table 2 shows the correlation results and mean square errors using neural networks. Figure 1 shows the bar chart chart of the mean square errors and the corresponding network structure revealing that structure 10 had the best performance. Figure 2 Shows the plot of electrode potentials versus exposure time for the steel samples Immersed in 1M NaCl solution and protected with different aluminium anodes. It shows that the steel sample protected with Al-Zn(0.5%)- Sn(0.1%) had the best performance of the three anodes throughout the experiment run. Figure 3 shows the plot of electrode potentials versus exposure time for the steel samples immersed in 0.5M NaCl solution. It shows that the steel sample protected with Al-Zn(10%)-Sn(0.1%) performed best of the three anodes.Figures 4 and 5 show a comparison between the experimental and modeled potential values for the low carbon steel sample in 1M NaCl solution.
3.2. Discussion of Results
3.2.1. Effect of Exposure Time on Potential
- Within the first 72 hours of the experiment the low carbon steel samples protected with Al-Zn-Sn anodes with varying compositions gave negative potentials. The steel sample protected with Al-Zn(10%)-Sn(0.1%) had a more negative potential of up to -0.97V after about 350 hours of exposure in 1M NaCl and -0.73V after 300 hours in 0.5M NaCl though the anode showed some form of fluctuations possibly due to uneven corrosion[7]. The more electronegative values of low carbon steel show that the Al-Zn(10%)-Sn(0.1%) anode is corroding quite fast in 1M solution. However the steel sample protected Al-Zn(5%)-Sn(0.1%) had a less negative potential of -0.87V after 350 hours of exposure, implying that the anode is not corroding as fast as that containing 10% Zn and hence a better anode material in 1M NaCl solution and that with 5% Zn performing better in 0.5M NaCl.
|
3.2.2. ANN Modeling Results
- From the results of the performance of twelve network structures are presented in table 2 and fig 1 it is evident that of all twelve structures used the 4-10-1 structure had the best R-value for test data set (0.850602), training data set (0.0799574), and validation data set (0.840233). The accuracy of this prediction is further indicated by the low mean square error (MSE) which ranged from 0.0261294 to 0.0451663. The number of iterations for the five structures ranged from 9 to 24.
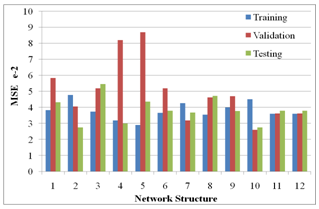 | Figure 1. Plot of Mean Square Error (MSE) Vs Network Structure |
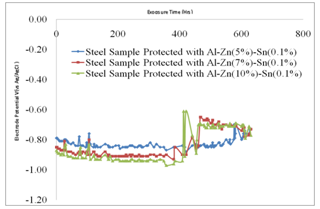 | Figure 2. Plot of Electrode Potentials versus Exposure Time for Low Carbon Steels Immersed in 1M NaCl solution |
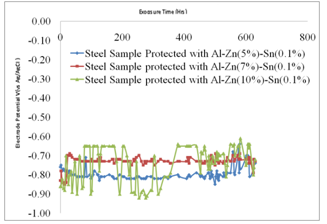 | Figure 3. Plot of Electrode Potentials versus Exposure Time for Low Carbon Steels Immersed in 0.5M NaCl solution |
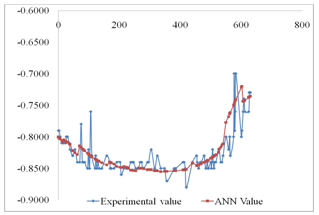 | Figure 4. ANN Modeling results compared with Experimental Results for steel protected with Al-Zn(5%)-Sn(0.1%) anode in 1M NaCl |
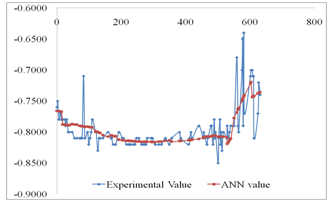 | Figure 5. ANN Modeling results compared with Experimental Results for steel protected with Al-Zn(5%)-Sn(0.1%) anode in 0.5M NaCl |
4. Conclusions
- 1) An effective neural network model for an Al-Zn-Sn sacrificial anode system was created using a standard commercial package with nearly all the trends noticed in the experimental data captured by the network.2) Artificial neural networks can be used to predict quite accurately the corrosion potential of low carbon steel in saline media.3) There is a need to conduct further experiments, use a larger data set and apply other neural network techniques to test their performance over the Levenberg Marquadt technique.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML