-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2012; 2(3): 39-43
doi: 10.5923/j.materials.20120203.01
Preparation and Characterization of Polyaniline-Co3O4 Nanocomposites via Interfacial Polymerization
Mahesh D. Bedre1, Raghunandan Deshpande2, Basavaraj Salimath3, Venkataraman Abbaraju1
1Materials Chemistry Laboratory, Department of Materials Science, Gulbarga University, Gulbarga Karnataka, 585106, India
2H.K.E.S’s Matoshri Taradevi Rampure Institute of Pharmaceutical Sciences, Sedam Road Gulbarga Karnataka, 585101, India
3Veeco-India Nanotechnology Laboratory, Jawaharlal Nehru Centre for Advanced, Scientific Research, Bangalore-64, India
Correspondence to: Venkataraman Abbaraju, Materials Chemistry Laboratory, Department of Materials Science, Gulbarga University, Gulbarga Karnataka, 585106, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Polyaniline (Pani) and Polyaniline-Co3O4 nanocomposites (PCO) were prepared by employing interfacial polymerization using ammonium persulphate as an oxidizing agent. The formations of regular nanocomposite materials were studied by Fourier transform infrared (FTIR) spectroscopy and XRD techniques. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images were conducted to characterize the morphology. Thermo-gravimetric (TG) and differential thermal analysis (DTA) were carried out study the thermal stability of the resulting composites. The nanocomposites were weakly ferromagnetic at room temperature. Formation of conducting emeraldine salt form was concluded by electrical conductivity.
Keywords: Interfacial Polymerization, Polyaniline, Co3O4 Nanocomposites, Conductivity
Article Outline
1. Introduction
- Magnetic nanoparticles (NPs) offer prodigious potential for applications not only as catalysts or energy storage devices, but also in biomedicine, as contrast enhancement agents for magnetic resonance imaging, or for drug delivery. The same characteristics that make cobalt-based nanoparticles highly attractive and stimulate the researchers to make cobalt based functional nanoparticles. In this context, we synthesized and characterized cobalt oxide nanoparticles. Believing that the characterization of nanoparticles is relevant for understanding their biological activity in future, we analyzed them using various instrumental techniques. It is reported that the biological effects could be due to a potential release of cobalt ions, we evaluated spontaneous dissolution in different media.Conducting polymer composites is some suitable composition of a conducting polymer with one or more inorganic nanoparticles so that their desirable properties are combined successfully. Over the last few years, conducting polymer composites have been studied with growing interest because of their numerous applications in various electrical and electronic devices[1-3]. Materials based on cobalt oxides have attracted a great interest in view of their technological and fundamental scientific importance[4]. In this paper, we report the preparation and characterization of polymer-cobalt oxide nanocomposites synthesized by interfacial polymerization method. Unlike the previous reported methods the formation metal oxide nanocomposites without using any surfactants block copolymer templates[5-10], where the metal oxide is introduced during the polymer synthesis, where metal oxide is associated with the polymer chain during polymer synthesis. In this method, no further doping is required after polymer synthesis and uniform distribution of metal oxide within a polymer matrix without the aid of any surfactant.Polyaniline is the only conducting polymer whose electrical properties can be controlled suitably by charge-transfer doping and/or protonation. Inorganic–organic composite materials are important due to their extraordinary properties, which arise from the synergism between the properties of the components. There are several routes to these materials, but probably the most prominent one is the incorporation of inorganic building blocks in organic polymers. These materials have gained much interest due to the remarkable change in properties such as mechanical[11], thermal[12-15], electrical[16], and magnetic[17] compared to pure organic polymers.Most of the efforts are based on well-known macro- or micro composites between inorganic moieties and organic polymers, and expand ideas from this microcosm to a new class of materials, called nanocomposites[18]. Expressions like ‘nanoparticles’ and ‘nanocomposites’ seem to be very trendy, and are often misused in the literature in the sense of advertising for systems which do not properly fall under the label ‘nano’. In view of inappropriate use of the term nano, there is a strong need to understand the mechanism of the formation of such materials. Important aspects of the chemistry involved in the formation of these systems are uniformity, phase continuity, domain sizes and the molecular mixing at the phase boundaries, which all have a direct influence on optical, physical, and mechanical properties[19].
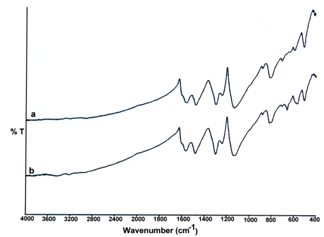 | Figure 1. (a-b) FTIR Spectrum of Polyaniline-Cobalt oxide nanocomposites |
2. Experimental
2.1. Materials
- All the chemicals and reagents used were of analytical grade. Double distilled water was used throughout the work. Prepared Cobalt oxide is used for the preparation of polymer composites[22] and ammonium persulphate was purchased from Qualigens; Aniline was double distilled before use and Hydrochloric acid was purchased from Aldrich chemicals. Polyaniline (Pani) and polyaniline-Cobalt oxide nanocomposites of different compositions (Pani-Co3O4) have been synthesized by employing interfacial polymerization method using ammonium persulphate as an oxidizing agent.
2.2. Preparation of Methods
- Pani-Co3O4 nanocomposites were prepared with different weights of Co3O4 (0.1, 0.2 and 0.3 g here after called as PCO1, PCO2 and PCO3 respectively.) The details of the method are as follows: 0.5 g of aniline is dissolved in 20 ml of CHCl3. 0.1 M ammonium persulphate is dissolved in 1.0 M HCl & the Co3O4 same is slowly added to the above mixture of aqueous and organic phase. After 5 min, dark-green formed slowly at the interface and then gradually diffused into the aqueous phase. After 24 hr, the entire aqueous phase was filled homogenously with dark-green color film, organic layer observed shows orange color due to the formation of aniline oligomers. The aqueous phase was then collected, and washed with ethanol and water to remove the unreacted aniline. The residue of polymer thus obtained is purified and dried in a vacuum oven at 40℃ for 36 hr. The dried polymer composite sample is used for the structural characterization. The same is further used for thermal and conductivity studies.
2.3. Characterization Techniques
- The powder X-ray diffraction patterns were recorded on a JEOL JDX-8P diffractometer using CuKα radiation (1.54 Å) at 30 kV. The Fourier transform infrared (FTIR) spectra of the samples were recorded on a Perkin-Elmer FT-IR (Model No. 1000) in the range 4000-400 cm-1 at a resolution of 4 cm-1. Bright field transmission electron microscopy image was obtained from JOEL 100 CX operated at 190 KeV. Thermo gravimetric (TG) and differential thermal analysis (DTA) experiments were performed using NETZSC H STA 409 PC. TG and DTA data were obtained at a heating rate of 10℃/min up to 900℃ under Argon atmosphere. Two probe D.C. electrical conductivity measurements were carried out using a Keithely high precision multimeter on pressed pellets of 1 c m thickness and 1 c m diameter.
3. Results and Discussion
3.1. FTIR Spectroscopy Studies
- Figure 1 (a-b) shows FTIR spectra of the polymer nanocomposite samples. The bands at 1563 and 1481 cm are attributed to C=N and C=C stretching mode of vibration for the quinonoid and benzenoid units of Pani. The peaks at 1300 and 1236 cm-1 are assigned to C– N stretching mode of benzenoid ring. The peak at 1239 cm-1 is the characteristic of the conducting protonated form of Pani[23,24]. The bands in the region 1000– 1110 cm-1 are due to in plane bending vibration of C– H mode. The band at 820 cm-1 originates out of plane C– H bending vibration. FTIR spectrum of the polymer composites shows two peaks at 665 and 703cm-1; which are attributed to the presence of cobalt oxide in the polymer nanocomposite (Figure 1.b). These two peaks closely match the reported values of the optical vibration modes of Co3O4[25].For the Pani-Co3O4 composites its IR spectrum is almost identical to that of the pure pani but all bands shift slightly towards red (lower frequency side), and the intensity ratio of quinonoid band has also changed. These results indicate that some interactions (kind of weak Vander Waals force of attraction) exist between pani and nano Co3O4.
3.2. X-ray Diffraction Studies
- The crystallinity and chain packing of the synthesized polymer-composites were examined by X-ray diffraction analysis. The Figure 2 (a-b) shows the characteristic of Pani[26] with other four main peaks at 2θ= 31.950, 38.30, 45.50 and 65.50 correspond to (220), (222) (400) & (440) matching with the JCPDS pattern of Co3O4 nanoparticles (JCPDS-78-1970).
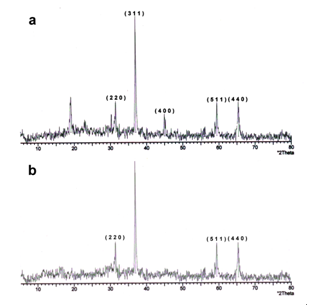 | Figure 2(a-b). XRD pattern of Polyaniline-Cobalt oxide nanocomposites |
3.3. Scanning Electron Microscopy
 | Figure 3. (a-b): SEM images of Polyaniline Cobalt oxide at low and high magnifications |
3.4. Transmission Electron Microscopy
- Transmission electron micrograph (TEM) images for the representative Pani-Co3O4 composite (Pani-CO1) is shown in Figure.4. Which shows the typical dark filed TEM image of the as synthesized Pani-Co3O4 nanocomposites. The TEM image clearly shows agglomerated irregular dark cloudy shaped to be nearly 100 nm in diameter sized Pani-Co3O4 nanocomposites.
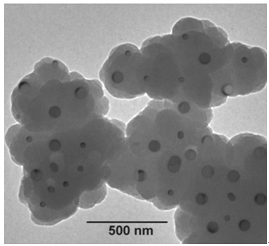 | Figure 4. TEM image of Polyaniline-Cobalt oxide nanocomposite |
3.5. Thermal Analysis
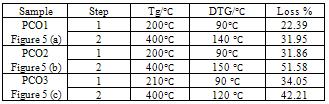
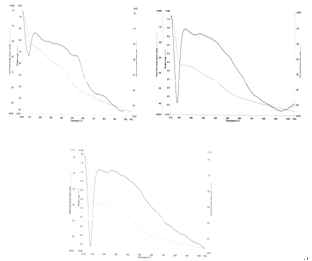 | Figure 5. (a-c) TGA of Polyaniline-Cobalt oxide nanocomposites |
|
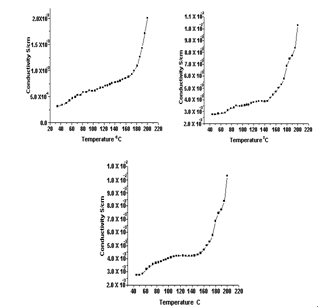 | Figure 6. (a-c) D.C Conductivities of Polyaniline-Cobalt oxide nanocomposites |
4. Conclusions
- The synthesis of Pani-Co3O4 composites (about 100 nm in diameter) were achieved by interfacial polymerization of aniline. SEM shows the formation of clusters and TEM shows the incorporation of Co3O4 into the polymer. The evidences from FTIR and XRD indicate that there is some interaction between Pani and cobalt oxide (weak Vander Waals force of attraction). TG analysis suggests that the thermal stability of Pani-Co3O4 composites is higher than that of pure Pani is ascribed to the interaction between Pani and metal oxide. The nanocomposites were weakly ferromagnetic at room temperature which has many advantages as optical and electrical nanodevices and nanosensors, bio-medical and optoelectronic devices etc.
ACKNOWLEDGEMENTS
- We acknowledge Department of Science and Technology (DST), India (Grant No. SR/S1/PC-10/2005), New Delhi, UGC minor research project, MRP(s)-241/2005 (Xth plan) KA GU 028/UGC-SWRO and UGC Innovative Programme in Materials Chemistry, New Delhi (D.O.No.F.14-4/2001 (Innov. Policy/ASIST)) for its financial assistance. We thank Sri. Jagannathrao Deshpande for editing works.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML