-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2012; 2(1): 32-36
doi: 10.5923/j.materials.20120201.06
Magnetic Susceptibility Measurements on Fly Ash Admixtured Cement Hydrated with Groundwater and Seawater
R. Gopalakrishnan 1, S. Barathan 2, D. Govindarajan 2
1Department of Physics, SRM University, Kattankulathur -603 203. Tamilnadu, India
2Department of Physics, Annamalai University, Annamalai Nagar, 608 002, Tamilnadu, India
Correspondence to: R. Gopalakrishnan , Department of Physics, SRM University, Kattankulathur -603 203. Tamilnadu, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The present works reports the effect of Flyash on the properties of Portland cement hydrated with ground water and seawater through magnetic susceptibility study. Cement pastes containing 0, 10, 20, 30% replacement of flyash with cement and in a Water cement ratio (W/C) ratio of 0.4 have been prepared. The magnetic susceptibility at different hydration ages has been determined by Faraday Curie balance method and this has been correlated to changes in setting time and compressive strength measurement. The observed result shows that, irrespective of water the magnetic susceptibility increases with increasing flyash percentage replacement level in cement.
Keywords: Magnetic susceptibility, Cement, Fly ash, Setting time, Seawater
Cite this paper: R. Gopalakrishnan , S. Barathan , D. Govindarajan , "Magnetic Susceptibility Measurements on Fly Ash Admixtured Cement Hydrated with Groundwater and Seawater", American Journal of Materials Science, Vol. 2 No. 1, 2012, pp. 32-36. doi: 10.5923/j.materials.20120201.06.
Article Outline
- Cement nomenclature:C = CaO; S = SiO2; H = H2O; C3S = 3CaO. SiO2; C2S =2CaO. SiO2; C3A = 3CaO. Al2O3C4AF =4CaO.Al2O3.Fe2O3; CH = Ca(OH)2
1. Introduction
- Flyash is generated in a power plant that uses bituminous and anthracite coal as a power source. The amorphous SiO2 content of this ash is high and it can be accepted as a pozzolanic material. Flyash is finer than Portland cement. It is known that fine pozzolans improve the properties of cement based materials due to their pozzolanic properties and filler effect. Reuse of flyash has been widely promoted and applied in many engineering applications and it offers cost reduction, energy savings, superior quality and also one way to reduce green house effect and global warming.The hydration mechanism of cement and mineral admixtured cement using X-ray diffraction analysis, Scanning electron microscopy, Differential thermal analysis, Fourier transform infrared spectroscopy, Nuclear magnetic resonance and Dielectric measurements was explained by number of authors’[1-6].During hydration of cement, the reaction products vary with time from one phase to another. The magnetic constituents of Portland cement and/or the reaction productsacquire magnetic properties due to their alignment with the external field and there is every possibility of change in susceptibility. Susceptibility (χ) measurements are used to detect the solubility limit of iron in different forms in C3A[7]. Beata J. Goluchowska[8] reported magnetic susceptibility of cement dusts depends on the iron content in raw materials, additives and fuels introduced into the kilns in which clinker in backed. According to David et al,[9] clay minerals have higher amount of oxides and hence this factor enhanced the susceptibility values. The magnetic susceptibility is an important physical property and it gives the analysis and/or chemical properties of cementitious materials. The magnetic susceptibility measurements of different water on cement hydration were studied by Govindarajan[10] with the help of Faraday Curie balance. They have reported that the cement mixing with distilled water has the highest value of susceptibility and then groundwater and then seawater.In India, due to the climate change and natural calamities (floods, delay of monsoon, tsunami and earthquake etc.,) the potable water quality and the availability of potable water are scarce commodity. Under these circumstances using other alternatives like rain water, river water and one of the natural potential i.e., seawater can also be think off, provided it is harmless.The influence of different waters on cement hydration was studied by Barathan et al.,[11] with the help of X-band Microwave technique. They have reported that the cement mixing with seawater has the highest value of dielectric constant (ε) and conductivity (σ) and then groundwater and then distilled water. According to Manu Santhanam et al.,[12] the high Chloride concentration of seawater could have played an important role by binding the C3A to form chloroaluminated compounds, such as Friedel’s salt and also by lowering the expansive potential of ettringite.To the knowledge of authors’, no magnetic susceptibility studies have been reported so far on hydration of cement admixtured with fly ash treated groundwater and seawater at different hydration periods. Several magnetic methods are available for measuring magnetic susceptibility but Faraday-Curie balance is very sensitive and suitable method to measure susceptibility of even a very small quantity of sample. In the present study, the authors’ present magnetic susceptibility measurements on cement and fly ash admixtured cement paste hydrated with groundwater and seawater at different hydrated time duration using Faraday-Curie balance available in Material Science Laboratory, Department of Physics, Annamalai University, Tamilnadu, India.
2. Materials and Methods
- Fly ash and Ordinary Portland cement (OPC) were commercial one. The percentages of oxides in the Ordinary Portland cement was found out to be CaO; 63.32, SiO2; 21.70, Al2O3; 5.40, Fe2O3; 3.40, MgO; 2.69, SO3; 2.70 and Loss on ignition was 0.79. The percentages of oxides in the flyash was found out to be CaO; 1.95, SiO2; 49.02, Al2O3; 32.03, Fe2O3; 7.14, MgO; 1.7, SO3; 1.2 and Loss on ignition was 1.58. The ground water (GW) and sea water (SW) were analysed using standard procedure recommended by Tamilnadu Water Supply and Drainage Board, Cuddalore, Tamilnadu, India (Table 1).
2.1. Preparation of Samples for Susceptibility Measurements
- The samples were used in the received form without any further modification. Groundwater and seawater were used for the preparation of the cement paste. The water-cement (W/C) ratio was 0.4 for all the samples. 10 grams of ordinary Portland cement was weighed and 4cc of water was added to the cement, so that the water cement ratio is 0.4 and made to a paste by gentle mixing. The flyash (FA) was mixed in the proportion of 10, 15, 20, 25 and 35 weight percent of Portland cement samples. Sample was then hydrated for various times. The hydration reaction was stopped at necessary different time duration like ½ hr, 1 hour, 2 hours, 4 hours, 6 hours, 1 day, 2 days, 4 days, 1 week, 2 weeks and 4 weeks by using acetone[13]. The solids were then allowed to dry in air to complete evaporation of the acetone. The dried solids were then oven dried at 110 0 C for 1 hour to remove the free or evaporable water. The dried samples were powdered using agate mortar. All the samples were ground for ½ hour and the grain size was maintained even. Finally the samples were stored in a dessicater. The powdered samples were weighed and then filled up in the capsule tightly. The sample was allowed to the sample holder and was used as the specimen for the curie balance. This procedure was repeated for different hydrated sample and hydrated to a time duration of ½ hour, 1 hour, 2 hours, 4 hours, 6 hours, 1 day, 2 days, 4 days, 1 week, 2 weeks and 4 weeks.
2.2. Setting Time and Compressive Strength Measurements
- Setting time, Compressive strength were measured on fly ash admixtured cement (0, 10, 15, 20, 25and 35 wt %) in a water to cement ratio of 0.4 treated with groundwater and sea water. Setting time was measured using a Vicat apparatus and the compressive strength were determined using Compression Testing Machine (Techno- Science Trading Corporation, New Delhi, India) set up available in Department of Structural Engineering, Annamalai University, India, and using standard procedure[14] and shown in Table 2.
|
|
2.3. Experimental details for susceptibility measurements
- Fig 1. Shows the Faraday-Curie balance along with Heyding’s type pole pieces[15].
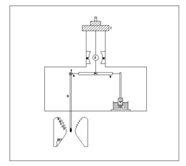 | Figure 1. Faraday Curie balance |
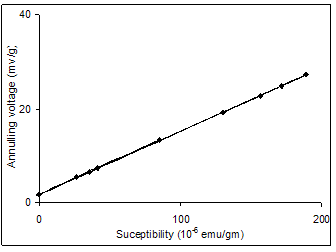 | Figure 2. Calibration graph |
3. Results and Discussion
- The susceptibility value of the anhydrous cement was observed to be 91.028×10-6emu/gm. This value χ>0 indicates the cement samples are paramagnetic. The positive paramagnetic susceptibility of the anhydrous cement may be considered as a resultant of the contribution from all the impurity ions and especially Fe2O3 contained in C3A and C4AF phases[17]. Graphs are drawn between the observed susceptibility values and different hydrated time duration for cement and flyash admixture cement treated with GW (Fig 3) and SW (Fig 4) in a non-uniform scale.
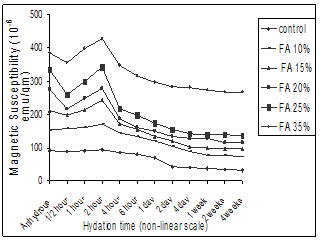 | Figure 3. Magnetic susceptibility vs. Different hydration time duration of flyash admixtured cement paste treated with groundwater. |
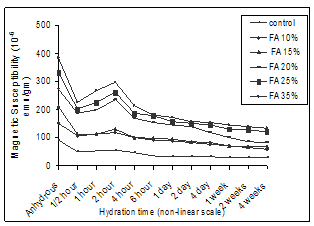 | Figure 4. Magnetic susceptibility vs. Different hydration time duration of flyash admixtured cement paste treated with seawater. |
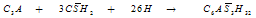 | (1) |
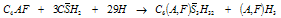 | (2) |
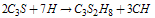 | (4) |
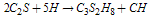 | (5) |
4. Conclusions
- The results can be summarized as follows1. The magnetic measurement is a good tool to study the chemical reactions of water and fly ash on cement hydration. As the weight percentages of fly ash increases, the susceptibility value also increases. This is due to the fly ash having a higher iron content and presence of more magnetic minerals.2. The high pozzolanic activity of fly ash increases the magnetic susceptibility of the cement with an increase in percentage of flyash replacement level.3. The quantitative value of magnetic susceptibility depends on the quality of water. As seawater contains more diamagnetic impurities, it decreases the magnetic susceptibility values when compared to groundwater treated samples.4. At early stage, the magnetic susceptibility values are high because the Portland cement dissolves, reacts greatly and forms hydration products, which disperses easily. After 2 hours, as the cement gel particles start to condense and thicken, the susceptibility values decreases as compressive strength increases. This trend is same irrespective of all samples when mixed with groundwater and sea water. 5. In the early stages, the reaction of fly ash and seawater accelerates the cement hydration which shortens the setting time and enhances the compressive strength, when compared to groundwater. It is also evident that the mineral content in seawater plays a major role in setting time, compressive strength and magnetic susceptibility.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML