-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2012; 2(1): 22-25
doi: 10.5923/j.materials.20120201.04
Tartrazine Dye and Bovine Serum Albumin: the Influence of pH on Adsorption Process
Jackeline B. Brito , Gean P. S. Aguiar , Júlio C. J. Flores , Josmary R. Silva , Nara C. de Souza
Grupo de Materiais Nanoestruturados, Universidade Federal de Mato Grosso, Barra do Garças, Mato Grosso, Brazil
Correspondence to: Nara C. de Souza , Grupo de Materiais Nanoestruturados, Universidade Federal de Mato Grosso, Barra do Garças, Mato Grosso, Brazil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Multilayers from Tartrazine dye (Tart) alternated with bovine serum albumin (BSA) were successfully prepared and a uniform growth has been observed. The influence of two values of Tart solution pH (3 and 7.5) on adsorption process of this molecule on BSA monolayer at physiological pH was investigated using kinetics and isotherms of adsorption, as well as thermostimulated adsorption experiments. All effects found by the changing of the pH were explicated mainly taking into account the ionic attraction (Tart at pH 3) and ionic repulsion (Tart at pH 7.5) that occurs during the adsorption process of Tart onto BSA monolayer.
Keywords: Tartrazine Dye, Bovine Serum Albumin, Layer-By-Layer Deposition Method, Adsorption Process, Ph Influence
Cite this paper: Jackeline B. Brito , Gean P. S. Aguiar , Júlio C. J. Flores , Josmary R. Silva , Nara C. de Souza , "Tartrazine Dye and Bovine Serum Albumin: the Influence of pH on Adsorption Process", American Journal of Materials Science, Vol. 2 No. 1, 2012, pp. 22-25. doi: 10.5923/j.materials.20120201.04.
Article Outline
1. Introduction
- Various heath problems are associated to the dyes, which are components of a lot of industrial products. Among the dyes, Tartrazine is very popular because is used in foodstuffs, cosmetics, textile, and medicines[1]. It has been also associated to diseases such as hyperactivity[2], asthma[3], and thyroid cancer[4]. Many studies have been carried out concerned to the Tartrazine dye (Tart)[5-8]. In special, the adsorption process of Tart has been investigated because its important role in applications, such as color removal from industrial effluents[9-12].In this work we prepared Layer-by-Layer (LbL) films in order to investigate the interactions of Tart and proteins. Bovine serum albumin (BSA) was chosen because it is the most abundant protein in blood plasma, and works like a transport protein for several substances (dyes and bioactive molecules)[13,14]. The BSA has been focus of several studies for their properties, such as pH-dependent binding[15] and interactions with phospholipids[16]. Besides that, BSA and human serum albumin, HSA, have a similarity of approximately 80% of their primary sequences[17]. This means that BSA and HSA are homologous proteins that should have very similar biological functions. Thus, as the Tartrazine is associated with various health problems, an investigation about the adsorption process of the dye and albumin can be used as an approach to analyze the biological process. Although there are several works on Tart and also BSA, we are not aware of any report about the preparation of multilayer systems and study of pH influence on adsorption process of Tart and BSA using the layer-by-layer (LbL) method. In this paper, we have not only studied the multilayer growth of Tart alternated with BSA, but also investigated the effect of pH on adsorption process of Tart on BSA monolayer focusing on the possible interactions associated to this process.
2. Materials and Methods
- Tartrazine and BSA (fraction V, purity 96–100%) from Acros Organics were used as received. The experimental film fabrication procedures are essentially the same as those described by Decher[18]. To build the multilayers, Tartrazine was alternated with 0.5 g/L BSA diluted in ultrapure water. The pH of the BSA solution was adjusted to 7.5 (close to physiological pH) by adding appropriate amounts of ammonium hydroxide. The final BSA solution was completely clear. The rinsing solution was ultrapure water with pH adjusted to 7.5. The Tartrazine solution was prepared to concentration of 0.5 g/L by dissolution in ultrapure water. The pH of the Tartrazine and the rinsing solutions were adjusted to 7.5 (anionic solution) and 3 (cationic solution) by adding an ammonium hydroxide solution or HCl respectively. The films were adsorbed on BK7 optical glass and the cleaned procedures are essentially the same described by previous works[1,19] The adsorption of Tartrazine, which is proportional to absorbance, was monitored by measuring the UV–Vis absorption spectra with a double-beam Thermo Scientific spectrophotometer model Genesys 10.
3. Results and Discussion
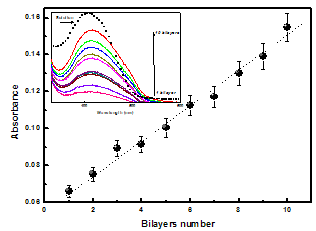
- We have employed the layer-by-layer (LbL) method to growth multilayers from BSA alternated with Tart (BSA/ Tart). Fig. 1 shows the absorbance at 425 nm versus the bilayer number, and an inset with the spectra of Tart solution and BSA/Tart (pH7.5/pH7.5) films. A linear increase in the absorbance was observed, suggesting that the same amount of material was adsorbed in each deposition cycle. The spectra (inset in Fig. 1) are essentially the same as those found in the literature for the Tartrazine[1,20]. The maximum absorption wavelength λmax of Tart molecules appears at 408 nm in aqueous solution (symbol line) and 425 nm in LbL films. These results reveal a λmax shift (Δλ = 17 nm) to red for the BSA/Tart bilayer in relation to the Tartrazine aqueous solution[1], indicating that the dye molecules join in J-aggregates with in-line transition dipoles[21]. The capacity of forming multilayers of the BSA and Tart reveals clearly that there are interactions between these two kinds of molecules, which permit the assembly of films[1].
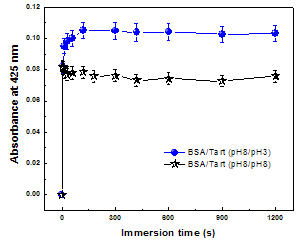 | Figure 2. Absorbance at 425 nm versus immersion time for BSA/Tart LbL film at different pH values. The solid line is a guide for the eye |
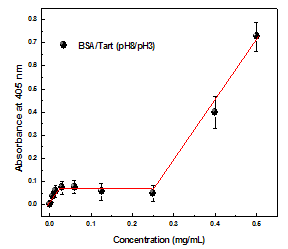 | Figure 3. Adsorption isotherm for Tart (pH 3) adsorbed on BSA monolayer (pH 8) with immersion time of 3 min. The solid line is a guide for the eye |
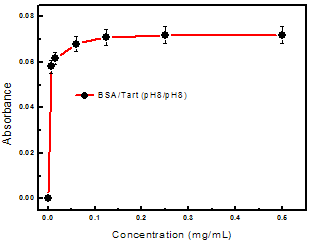 | Figure 4. Adsorption isotherm for Tart (pH 8) adsorbed on BSA monolayer (pH 8) with immersion time of 3 min. The solid line is a guide for the eye |
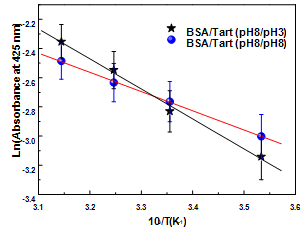 | Figure 5. Arrhenius plot for adsorption process of Tart (pH 8 and 3) adsorbed onto BSA monolayer (pH 8) |
4. Conclusions
- We have successfully prepared multilayer films from Tart and BSA and shown that the Tart interacts depends on the conditions of the solution pH used in the preparation of films. The adsorption process of Tart at pH 7.5 has not only adsorption rate faster, but also maximum adsorbed amount and activation energy lower than at pH 3. All this results was attributed mainly to ionic attraction associated to Tart at pH 3 and ionic repulsion associated to Tart at pH 7.5 when it is adsorbed on the BSA at pH 7.5. The possibility of forming multilayer films based on dyes and proteins can be used in investigations of biological sensors, whereas the knowledge of influence of pH on adsorption process of Tart on BSA monolayer may be useful to studies of interactions associated to health problems.
ACKNOWLEDGEMENTS
- This work was supported by CNPq and CAPES.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML