-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2011; 1(2): 133-138
doi: 10.5923/j.materials.20110102.22
Low Temperature Synthesis of the xBiScO3-(1-х)BaTiO3, x=0÷0.03 Ferroelectric System
Tatyana Karpova 1, Victor Vassil’ev 1, 2, Elena Vladimirova 1, 2, Vladimir Shur 3, Vera Shikhova 3, Vladimir Osotov 4, Alexander Nosov 4
1Ural Division of RAS, Institute of Solid State Chemistry, Ekaterinburg, 620041, Russia
2Ural Federal University named after the first President of Russia B.N. Yeltsin, Institute of
3Material Studies and Metallurgy, Ekaterinburg, 620002, Russia
4Ural Federal University named after the first President of Russia B.N. Yeltsin,
Correspondence to: Tatyana Karpova , Ural Division of RAS, Institute of Solid State Chemistry, Ekaterinburg, 620041, Russia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The ferroelectric ceramic of the xBiScO3-(1-х)BaTiO3, x=0÷0.03 system was prepared by solid state reaction synthesis at temperatures below 850℃. Solid solutions with tetragonal symmetry were formed. Doping with BiScO3 resulted in linear increase with x the unit cell parameters, maximum temperature of the dielectric constant and its maximum value. Room temperature measurements of ferroelectric properties revealed more complicated behavior on doping with optimum combination of ferroelectric properties for composition with x = 0.015.
Keywords: Oxides, Chemical Synthesis, Electron Microscopy, Dielectric Properties
Cite this paper: Tatyana Karpova , Victor Vassil’ev , Elena Vladimirova , Vladimir Shur , Vera Shikhova , Vladimir Osotov , Alexander Nosov , "Low Temperature Synthesis of the xBiScO3-(1-х)BaTiO3, x=0÷0.03 Ferroelectric System", American Journal of Materials Science, Vol. 1 No. 2, 2011, pp. 133-138. doi: 10.5923/j.materials.20110102.22.
Article Outline
1. Introduction
- The relaxor ferroelectrics with the perovskite ABO3 structure are characterized by smeared maximum at the temperature dependence of dielectric constant ε and its frequency dispersion in the region of phase transition[1]. Optimization of physical properties of these materials can be made by simple or complicated doping of the A or B positions of the perovskite structure[2-4]. The BaTiO3–based ferroelectrics systems attract permanent interest as Pb-free ecologically clean materials[5,6].An isovalent doping of B sites of the perovskite structure is usually used for tailoring the Curie temperature TC and lowering the temperatures of the tetragonal-orthorhombic and orthorhombic-rhombohedral phase transitions. For example, doping by Zr4+ and Sn4+ ions results in linear decrease of TC and increase of the temperatures of phase transitions[7-9]. Isovalent doping by ions of similar radius does not influence the charge states and no electrical fields appear. Isovalent doping of B-sites by ions with large (~20-30%) difference of ion radius results in appearance of random elastic fields and relaxor properties[9]. Heterovalent doping of B-sites substantially hinders the ordering processes in the perovskite structure and superstructures may appear.Heterovalent doping of A-sites in the BaTiO3 structure may result in both deterioration of ferroelectric order and appearance of relaxor behavior[10], and substantial variation of the temperatures of ferroelectric phase transitions. Substitution of Ba2+ by Bi3+ practically does not influence TC till doping level of 10%, however abnormal behavior of dielectric constant is observed at bismuth ion concentration of 2%. Such behavior is characteristic of the ferroelectric phase and characterized by substantial frequency dispersion [10].Simultaneous doping of both A- and B-sites in the BaTiO3 structure can be characterized by complicated mechanism of charge compensation and also results in substantial variation of ferroelectric properties: shift of the phase transition temperatures, relaxor behavior at small and low concentrations of doping ions, abnormal behavior of dielectric constant and other physical characteristics. Recent investigations of the xBiScO3-(1-х)BaTiO3 solid solutions[11,12] showed that they are characterized by high values of the piezoelectric constants and electromechanical coupling factor due to peculiarities of their phase diagram and existence of morphotropic phase boundary. The relaxor behavior is determined by disordering of heterovalent ions in the structurally equivalent sites of crystal lattice. At room temperature the BiScO3 compound has the monoclinic type of lattice symmetry and the question about existence of structural phase transitions still remains open[13]. At present BiScO3 is actively used as doping compound in various ferroelectric systems: BiScO3–PbTiO3 [14], (K0.5Bi0.5)TiO3–BiScO3–PbTiO3[15], BiScO3-(K0.475 Na0.475Li0.05)(Nb0.95Sb0.05)O3[16].According to[7,8] the xBiScO3-(1-х)BaTiO3 solid solutions with perovskite structure are formed for the concentration region 0≤x≤0.4. For 0≤x≤0.05 such solid solutions have tetragonal structure (the P4mm space group) with the degree of tetragonality linearly decreasing from 1.0103 (undoped BaTiO3) to 1.103 (x=0.5), such behavior is explained by cumulative effect from simultaneous c lattice parameter decrease and a lattice parameter increase. Note that in[8] calculations were made under assumption of equivalent of substitution of Ba and Ti by Bi and Sc ions, respectively, with equal values of the thermal disorder parameters. Coexistence of two phases – tetragonal (space group P4mm) and trigonal (space group R3m) - is observed for 0.05≤x≤0.20. The existence of phase with perovskite-like structure (space group Pm3m) was observed for х>0.2. It was shown that addition of BiScO3 may influence the ferroelectric properties of the xBiScO3-(1-х)BaTiO3 solid solutions [10]: for 0.02≤x≤0.10 substantial variations of the ferroelectric loop shape and remnant polarization were observed. However no data were obtained for lower doping concentrations.The principal problem of synthesis of Bi-containing oxide compounds using Bi2O3 as one of the starting components is its relatively low melting temperature 825℃[17]. Depending on the preparation method the influence of Bi volatility is minimized in different ways. When the samples are prepared by the solid state reactions method, long milling with subsequent anneal at relatively low temperature of 900℃ for the short period of time (2 h.)[18] is used. When the complex oxide compounds are prepared using molten salt synthesis with calcinations in sealed alumna crucibles at 1100℃ for 2 h with subsequent thorough washing with hot deionized water[19]. When the sol-gel method is used for synthesis, the samples are sintered at various temperatures from 1100 to 1150℃, for 1 h in air[20]. When the solid state reactions method is used at higher temperatures (1050-1320℃) and for longer durations (till 20 hours) the samples are prepared in a closed crucible with excess of Bi2O3 since more than two-thirds of its excess quantity can be evaporated during sintering[10]. The disadvantage of addition of excess quantities of Bi2O3 during sintering is in possible Bi incorporation into BaTiO3. Such Bi doping of BaTiO3 induces a decrease in Tc as was shown in[21].In this work the xBiScO3-(1-х)BaTiO3 solid solutions with 0.00 ≤ x ≤0.03 were prepared by solid state reactions method by heat treatment of stoichiometric mixtures of BaTiO3, Bi2O3, and Sc2O3 in the temperature interval from 500 till 850℃. The structural, dielectric, and ferroelectric properties of thus obtained bulk ceramics were studied.
2. Experimental
- Powder samples of xBiScO3-(1-х)BaTiO3, where x = 0, 0.010, 0.015, 0.020, 0.025, 0.030, were prepared by conventional solid state reactions method from reagent grade BaCO3, TiO2, Bi2O3, and Sc2O3, taken in stoichiometric proportions. The undoped BaTiO3 was synthesized by gradual increase of the sintering temperature from 800℃ to 1100℃ with intermediate grindings. The xBiScO3-(1-х) BaTiO3 samples were obtained by addition to BaTiO3 the stoichiometric amounts of Bi2O3 and Sc2O3 mixtures. These mixtures were first mixed in ethanol with the approximately equal quantities of barium titanate, then gradually the rest amount of barium titanate was added till the homogeneous mixture of the desired stoichiometry have been obtained. The mixtures were annealed with increase of the sintering temperature from 600℃ to 850℃ with intermediate grindings. Higher annealing temperatures were deliberately avoided due to volatility of Bi2O3. Total duration of sintering at the temperature of 850℃ was 46 hours. All anneals were carried out in air.X-ray diffraction analysis was performed at room temperature for structural analysis and lattice parameters determination using the Shimadzu XRD-7000 diffractometer with monochromatic CuKα radiation (λ=0,15418 нм) for the 2θ interval from 20˚ to 80˚ and scan speed 0.9˚/min. Phase identification was carried out using the JCPDSD-ICDD cards No.00-005-0626, and No.01-075-3569. Structural parameters were calculated using the FullProf Suite (version 2.00) software. In order to validate correctness of utilization of X-ray diffraction analysis for structural characterization of the xBiScO3-(1-х)BaTiO3 solid solutions with small doping levels (in our case x was less than 0.030) special calibration measurements have been made on the unannealed mixtures of BaTiO3 with the corresponding quantities of Bi2O3 and Sc2O3. The surface topography of the samples was studied using the JEOL JSM-6390LA scanning electron microscope with X-ray dispersive microanalysis. The size distribution of powder particles was measured using the Horiba partica LA-950V2 laser analyzer. For electrical measurements the powders were cold isostatically pressed at 15 MPa and disk-shaped bulk samples with typical dimensions of in diameter and 1÷3 mm in thickness were annealed at 850℃ for 6 h. in air. The density of bulk samples was determined by weighing in liquid. For electrical measurements on bulk pellet samples the sintered pellets were polished to achieve parallel, smooth surfaces, and electrodes were made by sintering of the Ag-containing paste at the temperature of 850℃ for 10 min in air. Dielectric measurements were performed from 30 to 190℃ in custom designed furnace with Actacom AM-3001 LCR meter. Ferroelectric hysteresis loops were measured at room temperature under the action of triangle ac field with frequency 1 Hz and amplitude up to 33 kV/cm. Pulses of the switching electric field were generated by DAC card PCI-6251 with LabVIEW-based software and amplified by high voltage amplifier TREK 20/2℃. The switching current data were recorded by measuring the signal on the serious resistor of 20 kΩ. The switching charge was obtained by digital integration of the current data.
3. Results and Discussion
- Figure 1 shows the X-ray diffraction patterns recorded at room temperature. Their analysis permitted to conclude that the solid solutions are formed and all samples have the tetragonal symmetry (the space group P4mm), which correspond to the known data[14,15].
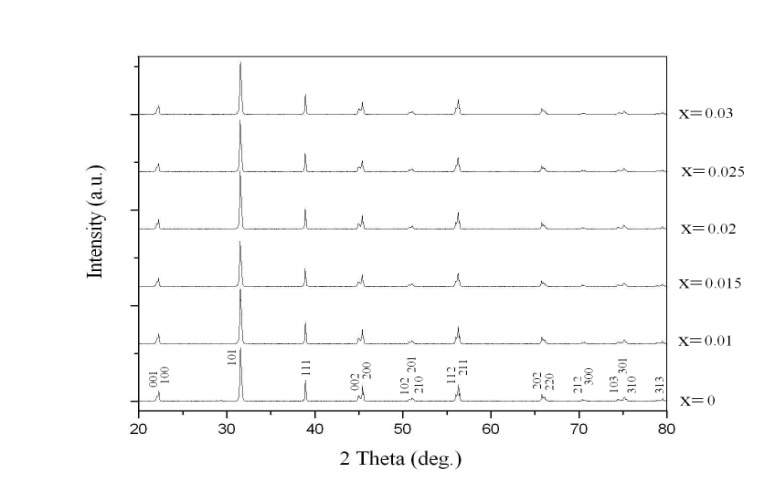 | Figure 1. X-ray diffraction patterns of the xBiScO3-(1-х)BaTiO3 samples |
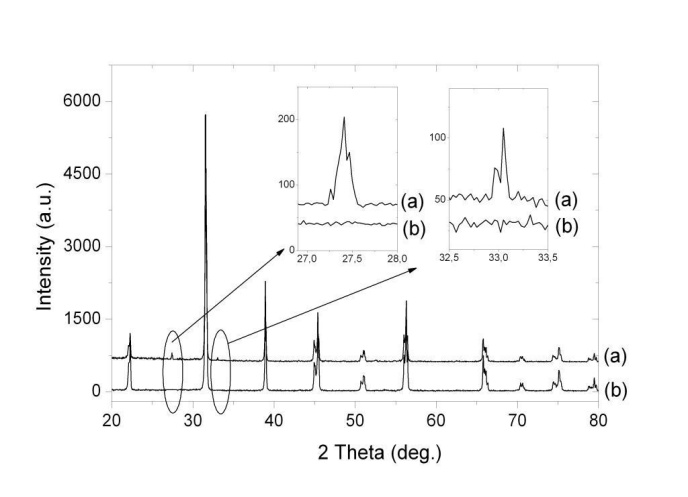 | Figure 2. X-ray diffraction patterns of the xBiScO3-(1-х)BaTiO3, x=0.01, just after mixing (a) and after annealing (b) of BaTiO3 with the corrsponding quantities of Bi2O3 and Sc2O3 |
 | Figure 3. SEM images of the 0.03BiScO3-0.97BaTiO3 sample surface. Backscattered image (a); secondary electron image (b) |
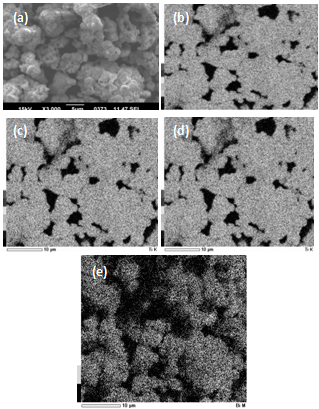 | Figure 4. SEM-image of the 0.03BiScO3-0.97BaTiO3 sample surface (a)with the results of EDX analysis: spatial distribution of the Ba element (b); spatial distribution of the Ti element (c); spatial distribution of the Sc element (d); spatial distribution of the Bi element (e) |
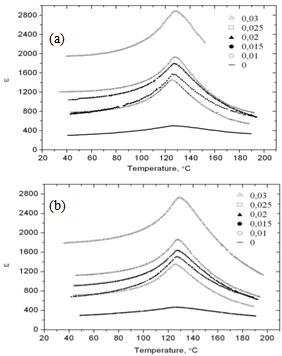 | Figure 5. Temperature dependences of the dielectric constant for the xBiScO3-(1-х)BaTiO3 samples recorded at frequencies of 100 Hz (a) and 100 kHz(b) |
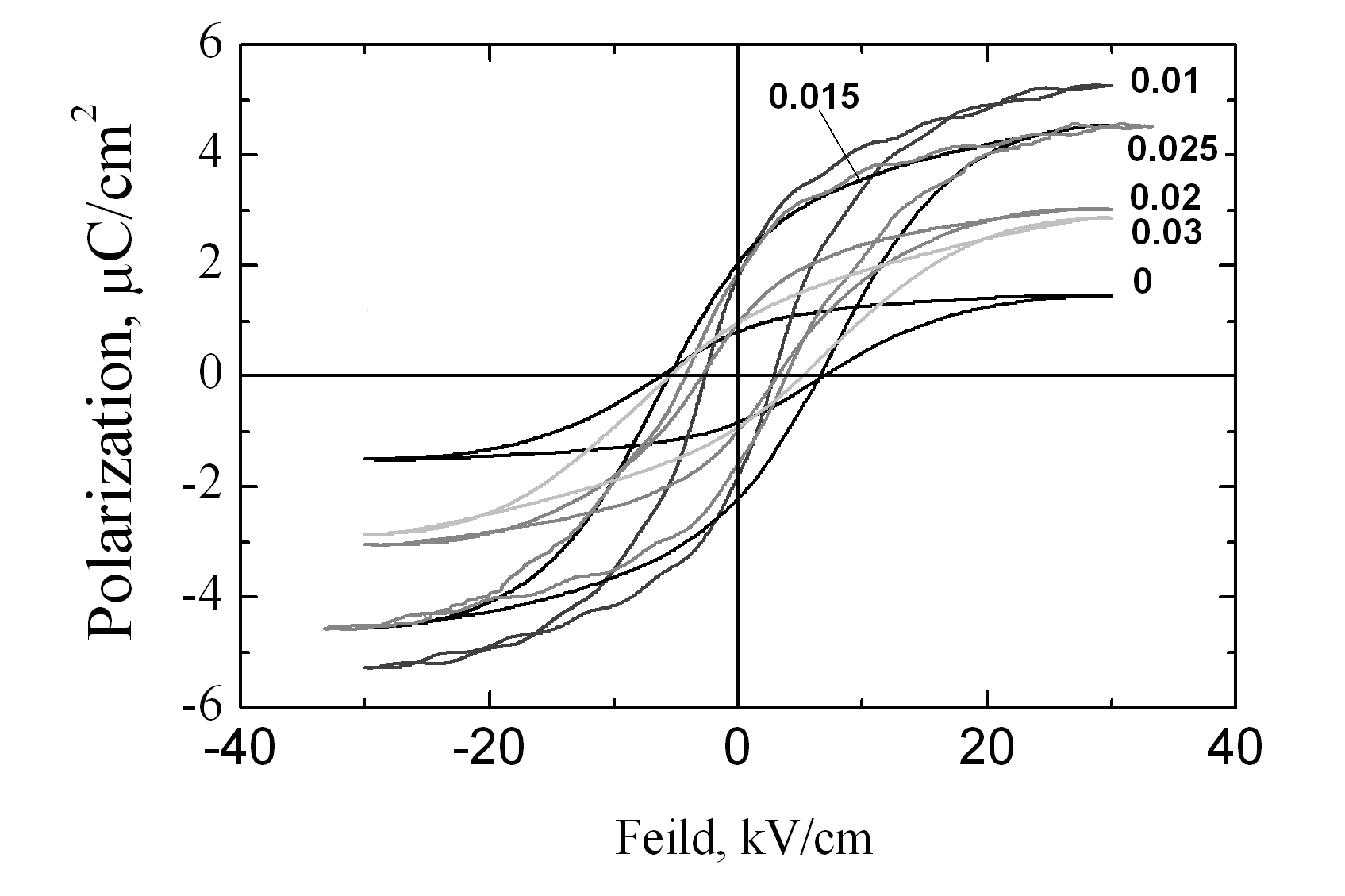 | Figure 6. Ferroelectric hysteresis loops for the xBiScO3-(1-х)BaTiO3, x=0÷0.03 samples |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
4. Conclusions
- The structural, dielectric, and ferroelectric properties of bulk ceramic xBiScO3-(1-х)BaTiO3 solid solutions with 0.00≤ x ≤ 0.03 were investigated. The samples were prepared by solid state reactions method at maximum heat treatment temperature of 850℃. Even at this low temperature it is possible to obtain stoichiometric solid solutions with tetragonal symmetry. Homogeneous distribution of principal elements, including Bi, over the sample surface was observed. Though the values of dielectric constant linearly increased with the doping level, optimum combination ferro- electric properties was found for x = 0.015.
ACKNOWLEDGEMENTS
- This work was supported by the Russian Foundation for basic Research (grant No. 08-02-99062_r_ofi), Program of the fundamental research of the Presidium of RAS No.21, Project of joint research of Ural and Siberian Branches of RAS No.09-S-2-1016, Program of the Department of physical sciences of RAS “Spin phenomena in solid-state structures and spintronics”.
References
| [1] | G.A. Smolensky, V.A. Bokov, V.A. Isupov, N.N. Kraynik, R.E. Pasynkov, A.I. Sokolov, N.K. Yushin. Physics of Ferroelectric Phenomena. Nauka, Leningrad. 1985 (in Russian) |
| [2] | Zou T., Wang X., Wang H., Zhong C., Li L., Chen I-W., 2008, Bulk dense fine-graine (1-x) BiScO3-xPbTiO3 ceramics with high piezoelectric coefficient, Appl. Phys. Lett., 93, 192913 |
| [3] | Aleksandrov S. E., Gavrilov G. A., Kapralov A. A., Smirnova E. P., Sotnikova G. Yu., Sotnikov A. V., 2004, Relaxer Ferroelectrics as Promising Materials for IR Detectors, Tech. Phys., 49(9), 1176-1180 |
| [4] | Lemanov V. V., Smirnova E. P., Zaіtseva N. V., 2009, Relaxors with Multiple Substitutions in Octahedral Sites of the Perovskite Structure V. V. Lemanov, Phys. of the solid state, 51(8), 1685-1690 |
| [5] | Gorev M. V., Bondarev V. S., Flerov I. N., Sciau Ph., Savariault J.-M., 2005, Heat Capacity Study of Double Perovskite-Like Compounds BaTi1-хZrxO3, Phys. of the solid state, 47(12), 2304-2308 |
| [6] | Jing Z., Yu Z.., 2003, Crystalline structure and dielectric properties of Ba(Ti1−yCey)O3, J. Mater. Sci. 38, 1057-1061 |
| [7] | Ravez J., Simon A., 2000, Non-stoicheometric perovskites derived from BaTiO3 with a Ferroelectric Relaxor Behaviour, Phys. Status Solid. A., 178, 793 |
| [8] | Simon A., Ravez J., Maglione M. 2004, The crossover from a ferroelectric to a relaxor state in lead-free solid solutions, J. Phys.: Cond. Matter., 16, 963-970 |
| [9] | Simon A., Ravez J., Maglione M., 2005, Relaxor properties of Ba0.9Bi0.067(Ti1−xZrx)O3 ceramics, Solid State Sci., 7 , 925-930 |
| [10] | Ogihara H., Randall A., Trolier- McKinstry S., 2009, Weakly Coupled Relaxor Behavior of BaTiO3–BiScO3 Ceramics J. Am. Ceram. Soc., 92 (1), 110-118 |
| [11] | Datta K., Thomas P.A., 2010, Structural investigation of novel perovskite-based lead-free ceramics: xBiScO3-(1-x) BaTiO3, J. Appl. Phys., 107, 043516 |
| [12] | Trolier- McKinstry S., Biegalski M.D., Wang J.., Belik A.A., Takayama-Muromachi E., Levin I., 2008, Growth, crystal structure, and properties of epitaxial BiScO3 thin films, Appl. Phys., 104, 044102-1−044101-7 |
| [13] | Yao Zh., Liu H., Liu Y., Chen L., Hao H., 2009, Structure and ferroelectric properties in (K0.5Bi0.5)TiO3–BiScO3–PbTiO3 piezoelectric ceramic system, Matter. Res. Bulletin, 44(7), 1511-1514 |
| [14] | Hungría M., Amorín H., Algueró M., Castro A., 2011, Nanostructured ceramics of BiScO3–PbTiO3 with tailored grain size by spark plasma sintering, Scripta Mater., 64(1), 97-100 |
| [15] | Li X., Zhu J., Wang M., Luo Y., Shi W., Li L., Zhu J., Xiao D., 2010, BiScO3-modified (K0.475Na0.475Li0.05)(Nb0.95Sb0.05)O3 lead-free piezoelectric ceramics, J. Alloys and Compounds, 499(1), L1-L4 |
| [16] | Chuprunov E.V., Khokhlov A.V., Faddeev M.A.. The Basics of Crystallography. Editorial board for physical and mathematical sciences. Moscow. 2004. 500p. (in Russian) |
| [17] | Weast R. C., Handbook of chemistry and physics, A Ready-Reference Book of Chemical and Physical Data. CRC Press, Boca Raton, FL, 1976 |
| [18] | Sun R., Wang X., Shi J., L. Wang. Appl., 2011, Dielectric and polar order behaviors of BaTiO3-Bi(Mg1/2Ti1/2)O3 ceramics., Appl Phys A, 104, 129–133 |
| [19] | Feng G. , Rongzi H., Jia-ji L., Yong-hong Y., Chang-sheng T., 2008, Effect of different templates on microstructure of textured Na0.5Bi0.5TiO3–BaTiO3 ceramics with RTGG method, J.Europ. Cer. Soc., 28, 2063–2070 |
| [20] | Cernea M., Andronescuc E., Radua R., Fochib F., Galassib C., 2010, Sol–gel synthesis and characterization of BaTiO3-doped (Bi0.5Na0.5)TiO3 piezoelectric ceramics, J. of Alloys and Compounds, 490, 690–694 |
| [21] | Bahri F., Simon A., Khemakhem H., Ravez, 2001, Classical or Relaxor Ferroelectric Behaviour of Ceramics with Composition Ba1−xBi2/3xTiO3, Phys.Status Solidi A, 184 (2), 459–464 |
| [22] | Yasuda N., Kato T., Hirai T., Mizuno M., Kurachi K., Taga I., 1994, Dielectric properties of BaTiO3, Sr- and Pb-substituted BaTiO3 ceramics synthesized through hydrothermal method, Ferroelectrics. 154, 331 |
| [23] | Damjanovic D., 1998, Ferroelecric, dielectric and piezoelectric properties of ferroelectric thin films and ceramics, Rep. Prog. Phys., 61, 1267–1324 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML