-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2011; 1(2): 98-102
doi: 10.5923/j.materials.20110102.16
Recyclable Copper Oxide Catalyzed Synthesis of N-Arylpyrroles from Arylhalides and Trans-4-Hydroxy-L-Proline
Sridhar. R , Yedukondalu. M , Chandrasekhar.N , Mandava V. Basaveswara Rao
Department of Chemistry, Krishna University, Machilipatnam, 521001, A.P, India
Correspondence to: Mandava V. Basaveswara Rao , Department of Chemistry, Krishna University, Machilipatnam, 521001, A.P, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
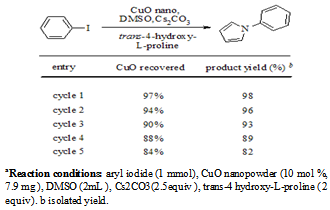
A facile and novel copper oxide nanoparticle catalyzed route has been developed for the synthesis of N-arylpyrroles, through tandem one-pot cross-coupling reaction of aryl halides and trans-4-hydroxy-L-proline, which is procured from natural source. This methodology is a first report of ligand free conditions and is a sustainable resource, via extrusion followed by redox amination. Catalyst used can be recovered and recycled up to five cycles with efficient activity.
Keywords: Aryl Halides, Cross-Coupling, Trans-4-Hydroxy-L-Proline, N-Arylpyrroles, Tandem –Reaction
Cite this paper: Sridhar. R , Yedukondalu. M , Chandrasekhar.N , Mandava V. Basaveswara Rao , "Recyclable Copper Oxide Catalyzed Synthesis of N-Arylpyrroles from Arylhalides and Trans-4-Hydroxy-L-Proline", American Journal of Materials Science, Vol. 1 No. 2, 2011, pp. 98-102. doi: 10.5923/j.materials.20110102.16.
Article Outline
1. Introduction
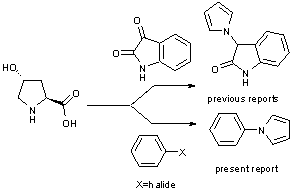
- Synthetic protocols utilizing biomass resources as an alternative feedstock devoid of fossil fuel resources are becoming more attracting these days due to the growing concern for sustainable chemistry. In this context organic synthesis is focused on the development of new synthetic methods to replace the existing starting materials made by synthetic reactions with simple synthons obtained from natural materials. The trans-4-hydroxy-L-proline is one such sustainable synthon which can replace pyrrole for synthesizing various pyrrole derivatives, since it is obtained from the renewable resource, collagen.1 Isolation of trans-4 hydroxy-L-proline from collagen is the most economical way of production1a and collagen is the main component of connective tissue and the most abundant protein in mammals.1b Various advantages associated with sustainable chemistry prompted us to explore trans-4-hydroxy-L-proline for the synthesis of variety of substituted pyrroles. Pyrroles are an important class of heterocycles obtained from nature having broad spectrum of biological profiles.2 They are constituents of many structural motifs showing antitumor, immunosuppressant, anti HIV, anti-inflammatory and antioxidant activities.3 Apart from this, they are also widely used as materials with versatile applications in material science.4 Several methods are reported for the construction of the pyrrole moiety. Amongst them, the pyrrole ring generation via a cascade reaction using trans-4-hydroxy-L-proline is of recent development.1 As such the N-arylatedpyrroles, whichhave been shown to be potent HIV inhibitors,3h are constructed via cross-coupling of pyrrole with aryl halides. We have envisaged the combination of the above two concepts and report the successful synthesis of novel one pot domino procedure to construct the N-arylpyrroles using aryl halides and trans-4-hydroxy-L-proline (Scheme 1).
 | Scheme 1. trans-4-hydroxy-L-proline as pyrrole synthon. |
2. Results and Discussion
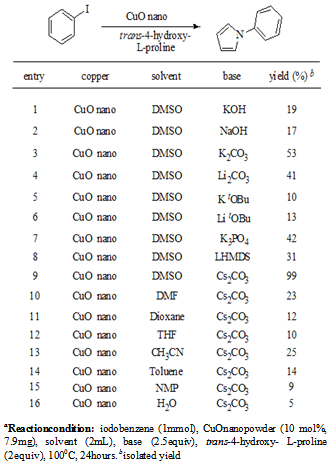
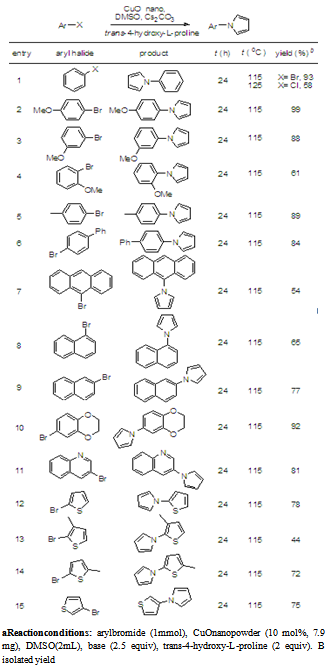
- We are successful in reacting iodobenzene and trans-4- hydroxy-L-proline in the presence of Copper Oxide nano- powder and potassium carbonate using with dimethylsulfoxide as a solvent at 100℃ yielding 53% of N-phenyl- pyrroles. An attempt is made in vain to improve the yields using various solvents and bases under the optimized conditions. When the reaction tried with various bases like potassium tert-butoxide, Lithium tert- butoxide, lithium carbonate, potassiumphosphate, Lithiumbis (trimethylsilyl) amide, sodium hydroxide, potassium hydroxide yield could not be increased. Similarly various solvents such as toluene, 1,4- dioxane, tetrahydrofuran, N-Methyl-2-pyrrolidone, and acet- onitrile were tried in vain to improve the yields as shown in Table 1. Out of all these attempts the best results were obtained by using copper oxide nanoparticles in dimethylsulfoxide as solvent and cesium carbonate as a base at 100℃ stirred for 24 hours. Having achieved the optimization of the reaction by suitable catalyst, base and solvent, we further explored the N-arylation with variety of aryl bromides. The reaction of arylbromides required 115℃ for the N-arylation. We extended the same reaction with various aryl bromides having different substituents at the ortho, para and meta positions.
|
|
|
3. Conclusions
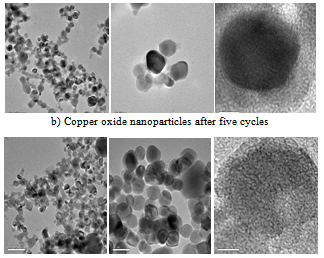
- In conclusion this is the first report of sustainable resource reaction of trans-4-hydroxy-L-proline, with various aryl halides, by a tandem one-pot cross-coupling reaction yields N-arylpyrroles via extrusion followed by redox amination.TEM images of native Copper oxide nanoparticles
4. Experimental Section
- Typical procedure for N-aryl pyrrole synthesis: A mixture of aryl halide (1 mmol), Copper oxide nanopowder (7.9 mg, 10 mol%), cesium carbonate (815 mg, 2.5 equiv), trans-4-hydroxy-L-proline ( 262 mg, 2 equiv ) were stirred in dimethylsulfoxide (2mL) under dry nitrogen atmosphere at respective temperature for 24h in a 25 mL round bottom flask equipped with a reflux condenser. The progress of the reaction was monitored by thin layer chromatography. The reaction mixture was allowed to cool to room temperature and was extracted with ethyl acetate (2 X 5 mL). The combined organic extracts were washed with water (2X5mL), dried over anhydrous sodium sulphate. The dried organic extracts were concentrated under reduced pressure and column chromatographed using hexanes/ethyl acetate as eluent to obtain the analytically pure product, which was characterized using 1H, 13C-NMR and elemental analysis.The spectral and analytical data are given for the molecules synthesized 1-phenyl-1H-pyrrole (table 2, entry 1): white solid, mp 58-61℃, 1H-NMR (300MHz, CDCl3): δ= 6.27 (t, 2H), 7.02 (t, 2H), 7.18- 7.20 (m, 1H), 7.35-7.40 (m, 4H). 13C-NMR (75MHz, CDCl3): δ = 110.4, 119.2, 120.4, 125.6, 129.3. Analitically calculated for: (C10H9N) C: 83.88, H: 6.34, N: 9.78, found C: 83.28, H: 6.26, N: 9.72.1-(4-methoxyphenyl)-1H-pyrrole (table 2, entry 2): white solid, mp 111-113℃ , 1H-NMR (300MHz,CDCl3): δ= 3.81 (s, 1H), 6.22 (t, 2H) ,6.88-6.91( m,4H ),7.25-7.27 (m, 2H). 13C-NMR (75MHz, CDCl3): δ = 55.4, 109.8, 114.5, 119.5, 122.1, 134.4, 157.5. Anal calcd for: (C11H11NO) C: 76.28, H: 6.40, N: 8.09; found C: 75.53, H: 6.33, N: 8.02.1-(3-methoxyphenyl)-1H-pyrrole (table 2, entry 3): colorless oil, 1H-NMR (300MHz, CDCl3): δ=3.82 (t, 2H), 6.89-7.05 (m, 3H), 7.28 (m, 3H). 13C-NMR (75MHz, CDCl3): δ= 55.39, 110.4, 110.8, 112.9, 119.4, 130.3, 141.9, 160.5. Analitically calculated: (C11H11NO) C: 76.28, H: 6.40, N, 8.09; found C: 75.78, H, 6.33; N, 7.981-(2-methoxyphenyl)-1H-pyrrole (table 2, entry 4 ): colorless oil, 1H-NMR (300MHz,CDCl3): δ =3.83 (s, 3H), 6.25(t, 2H), 6.90 (t, 2H), 6.97-6.99 (m, 2H), 7.18-7.27 (m, 2H) 13C-NMR (75MHz, CDCl3):δ = 55.8, 108.8, 112.3, 121, 122.1, 125.8, 127.5, 130.3, 152.8 Analitically calculated for: (C11H11NO) C: 76.28; H: 6.40; N: 8.09; found C: 75.59; H: 6.33; N: 8.03.1-p-tolyl-1H-pyrrole (table 2, entry 5): white solid, mp 82-85℃, 1H-NMR (300MHz, CDCl3): δ= 2.37 (s, 3H), 6.24 ( t, 2H), 6.97 (t, 2H), 7.15 (dd, 2H), 7.24 (dd, 2H). 13C-NMR (75MHz, CDCl3): δ =20.7, 110.1, 119.3, 120.4, 129.9, 135.3, 138.4. Analitically calculated for: (C11H11N) C: 84.04, H: 7.05, N: 8.91; found C: 83.30, H: 6.95, N: 8.84.1-(biphenyl-4-yl)-1H-pyrrole (table 2, entry 6): white solid, mp 183-188℃.1H-NMR (300MHz, CDCl3): δ = 6.3 (s,2H), 7.1 (s, 2H), 7.24-7.45 (m, 5H), 7.54-7.62 (m, 4H); 13C-NMR (75MHz, CDCl3): δ =110.5, 119.2, 120.7, 126.9, 127.4, 128.2, 128.9, 138.5, 139.5, 140.2. Analitically calculated for: (C16H13N) C: 87.64, H: 5.98, N: 6.39; found: C: 87.58, H: 5.91, N: 6.34.1-(anthracen-9-yl)-1H-pyrrole (table 2, entry 7): white solid, mp 149-153℃. 1H-NMR (300MHz, CDCl3): δ = 6.45 (s, 2H), 6.94 (s, 2H), 7.41-7.52 (m, 6H), 7.95-8.02 (d, 2H), 8.49 (s, 1H). 13C-NMR (75MHz, CDCl3): δ = 108.8, 123.4, 124.5, 125.6, 126.8, 127.2, 128.1, 129.2, 131.3, 133.4. Analitically calculated for: (C18H13N) C: 88.86, H: 5.39, N, 5.76; found: C: 88.79, H: 5.31, N: 5.69.1-(naphthalen-1-yl)-1H-pyrrole (table 2, entry 8): pale yellow oil, 1H-NMR (300MHz, CDCl3): δ= 6.32 (t, 2H), 6.93 (t, 2H), 7.42-7.51 (m, 1H), 7.81-7.88 (m, 2H). 13C-NMR (75MHz, CDCl3): δ = 108.9, 123.2, 123.3, 125.2, 126.5, 126.6, 127.8, 128,129.8, 134.2 Analitically calculated for: (C14H11N) C: 87.01, H: 5.74, N: 7.25; found C: 86.26, H: 5.66, N: 7.17.1-(naphthalen-2-yl)-1H-pyrrole (table 2, entry 9): white solid, mp 78-84℃. 1H-NMR (300MHz, CDCl3): δ= 6.31 (t, 2H), 7.14 (t, 2H), 7.27-7.51 (m, 3H), 7.54-7.57 (m, 1H ), 7.75-7.87(m, 4H). 13C-NMR (75MHz, CDCl3): δ = 110.6, 117.4, 119.5, 120.2, 125.6, 127.1, 127.6, 127.8, 129.6, 129.9, 131.4, 133.8. Analitically calculated for: (C14H11N) C: 87.01, H: 5.74, N: 7.25; found C: 86.12, H: 5.57, N: 7.14.1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-1H-pyrrole (table 2, entry 10): white solid, mp 94-97℃ 1H-NMR (300MHz,CDCl3): δ = 4.26 (m, 4H), 6.24 (t, 2H), 6.83-6.86 (m, 3H). 13C-NMR (75MHz, CDCl3): δ = 64.3, 64.5, 109.9, 113.8, 117.8, 135, 141.7, 143.9. Analitically calculated for: (C12H11NO2) C, 71.63; H, 5.51; N, 6.96; found C, 70.95; H, 5.42; N, 6.89.3-(1H-pyrrol-1-yl) quinoline (table 2, entry 11): off white solid, mp 69-74℃. 1H-NMR (300MHz, CDCl3): δ = 6.39 (t, 2H), 7.17 (t, 2H), 7.54-7.59 (t, 1H), 7.65-7.70 (t, 1H), 7.80-7.82 (d, 1H), 8.02-8.04 (m, 1H), 8.10-8.12 (d, 1H), 9.06-9.08 (d, 1H). 13C-NMR (75MHz, CDCl3): δ= 111.6, 119.4, 124.6, 127.7, 128.9, 129.5, 132.7, 144.6, 146.3. Analitically calculated for: (C13H10N2) C: 80.39, H: 5.19, N: 14.42; found C: 79.82, H: 5.09, N: 14.36.1-(thiophen-2-yl)-1H-pyrrole (table 2, entry 12): white solid, mp 75-78℃ 1H-NMR(300MHz, CDCl3): δ = 6.22 (t, 2H), 6.85 (t, 2H), 6.89-6.92 (m, 3H). 13C-NMR (75MHz, CDCl3): δ = 110.4, 115.5, 119.1, 121.2, 126.1, 150.6. Analitically calculated for: (C8H7NS) C: 64.39, H: 4.73, N: 9.39, S: 21.49; found C: 63.67, H: 4.64, N: 9.31, S: 20.631-(3-methylthiophen-2-yl)-1H-pyrrole (table 2, entry 13): colorless oil, 1H-NMR (300MHz, CDCl3): δ = 2.15 (s, 3H), 6.22 (t, 2H), 6.75-6.77 (m, 3H), 6.98-6.99 (m, 1H). 13C-NMR (75MHz, CDCl3): δ = 11.8, 109.5, 120.5, 123.2, 128.6, 135.6, 144.3. Analitically calculated for: (C9H9NS) C: 66.22, H: 5.56, N: 8.58; S, 19.64; found C: 65.45, H: 5.48, N: 8.51, S: 18.56.1-(5-methylthiophen-2-yl)-1H-pyrrole (table 2, entry 14): white solid, mp 87-91℃ 1H-NMR (300MHz,CDCl3): δ = 2.45 (s, 3H), 6.19 (t, 2H), 6.51-6.52 (m, 1H), 6.61-6.62 (m, 1H), 6.85 (t, 2H). 13C-NMR (75MHz, CDCl3): δ = 15.3, 110, 115.5, 121.2, 123.6, 133.7, 141.4. Analitically calculated for: (C9H9NS) C: 66.22, H: 5.56, N: 8.58, S: 19.64; found C: 65.75, H: 5.42, N: 8.47, S: 18.23.1-(thiophen-3-yl)-1H-pyrrole (table 2, entry 15):white solid, mp 99-101℃ 1H-NMR(300MHz,CDCl3): δ =6.21 (t, 2H), 6.95 (t, 2H), 7.02 (m, 1H), 7.14 (m, 1H), 7.31 (m, 1H). 13C-NMR (75MHz, CDCl3): δ =109.8, 119.7, 121.1, 126.2, 149.4. Analitically calculated for: (C8H7NS) C:64.39, H: 4.73, N: 9.39, S: 21.49; found C: 63.54, H: 4.57, N: 9.24, S: 20.16.1-(3-chlorophenyl)-1H-pyrrole (table 3, entry 1): pale yellow oil, 1H-NMR (300MHz, CDCl3): δ = 6.27 (t, 2H), 7.00 (t, 2H), 7.17 (d, 1H), 7.24 (d, 1H), 7.32 (m, 1H) ,7.38 (m, 1H). 13C-NMR (75MHz, CDCl3):δ110.9, 118.4, 119.2, 120.5, 125.5, 130.5, 135.1, 141.7. Analitically calculated for: (C10H8ClN) C: 67.62, H: 4.54, N: 7.89; found C: 66.93, H: 4.44, N: 7.83.1-(4-methoxyphenyl)-1H-pyrrole (table 3, entry 2): white solid, mp 111-113℃ , 1H-NMR (300MHz,CDCl3): δ= 3.81 (s, 1H), 6.22 (t, 2H), 6.88-6.91 (m, 4H ),7.25-7.27 (m, 2H). 13C-NMR (75MHz, CDCl3): δ = 55.4, 109.8, 114.5, 119.5, 122.1, 134.4, 157.5. Analitically calculated for: (C11H11NO) C: 76.28, H: 6.40, N: 8.09; found C: 75.67, H: 6.28, N: 7.95.1-(4-nitrophenyl)-1H-pyrrole (table 3, entry 3): white solid, mp 180-183℃. 1H-NMR (300MHz, CDCl3): δ= 6.37(t, 2H), 7.13 (t, 2H), 7.53 (d, 2H), 8.29 (d, 2H). 13C-NMR (75MHz, CDCl3): δ=112.5, 119.1, 119.4, 125.5, 144.6, 145.2. Analitically calculated for: (C10H8N2O2) C: 63.82, H: 4.28, N: 14.89; found: C: 63.24, H: 4.19, N: 14.83.1-p-tolyl-1H-pyrrole (table 3, entry 4): white solid, mp 82-85℃, 1H-NMR (300MHz, CDCl3): δ= 2.37 (s, 3H), 6.24 (t, 2H), 6.97 (t, 2H), 7.15 (dd, 2H), 7.24 (dd, 2H). 13C-NMR (75MHz, CDCl3): δ = 20.7, 110.1, 119.3, 120.4, 129.9, 135.3, 138.4. Analitically calculated for: (C11H11N) C: 84.04, H: 7.05, N: 8.91, found C: 83.30, H: 6.95, N: 8.84.1-(4-tert-butylphenyl)-1H-pyrrole (table 3, entry 5): white solid, mp 68-72℃ 1H-NMR(300MHz, CDCl3): δ = 1.35 (s, 9H), 6.25 (t, 2H), 6.99 (t, 2H), 7.36-7.41 (d, 2H), 7.24-7.30 (d, 2H). 13C-NMR (75MHz, CDCl3): δ = 31.3, 34.4, 110.1, 119.3, 120.2, 126.3, 138.3, 148.6. Analitically calculated for: (C14H17N) C: 84.37, H: 8.60, N: 7.03found C: 83.74, H: 8.51, N: 6.96.1-(4-(benzyloxy)phenyl)-1H-pyrrole (table 3, entry 6): white solid, mp 102-105℃ 1H-NMR(300MHz, CDCl3): δ = 5.01 (s, 2H), 6.23 (t, 2H), 6.91 (m, 1H), 6.95 (d, 1H), 6.97 (d, 1H), 7.25-7.28 (m, 3H) , 7.32-7.40 (m, 5H). 13C-NMR (75MHz, CDCl3): δ = 70.4, 109.9, 115.7, 119.6, 127.5, 128.6, 129.5, 134.7, 136.8, 156.8. Analitically calculated for: (C17H15NO) C: 81.24, H: 6.06, N: 5.62; found C: 80.71, H: 5.98, N: 5.54.1-(3,5-bis(trifluoromethyl)phenyl)-1H-pyrrole (table 3, entry 7): colorless oil, 1H-NMR (300MHz, CDCl3): δ= 6.37 (t,2H), 7.01 (t,2H), 7.72 (s,1H), 7.80 (s, 2H). 13C-NMR (75MHz, CDCl3): δ= 112.3, 118.8, 119.1, 120.0, 124.6, 132.9, 141.7. Analitically calculated for: (C12H7F6N) C: 51.63, H: 2.53, N: 5.02; found C: 50.95, H: 2.47, N: 4.95.1-(4-ethylphenyl)-1H-pyrrole (table 3, entry 8): off white solid, mp 67-70℃. 1H-NMR (300MHz, CDCl3): δ= 1.25 (t, 3H), 2.64 (q, 2H), 7.00 (t, 2H), 7.19 (d, 2H), 7.26 (d, 2H). 13C- NMR (75MHz, CDCl3): δ=15.8, 28.4, 110.3, 119.2, 120.7, 128.8, 138.9, 141.4. Analitically calculated for: (C12H13N) calculated C: 84.17, H: 7.65, N: 8.18, found C: 84.09, H: 7.55, N: 8.10.3-(1H-pyrrol-1-yl) pyridine (table 3, entry 9): colorless oil. 1H-NMR (300MHz, CDCl3): δ = 6.34 (t, 2H), 7.0 (t, 2H), 7.35 (m, 1H), 7.66 (m, 1H), 8.47 (m, 1H), 8.74 (m, 1H) 13C-NMR (75MHz, CDCl3): δ = 111.7, 119.1, 123.9, 127.3, 134.9, 142.2, 146.7. Analitically calculated for: (C9H8N2) calculated C: 74.98, H: 5.59, N: 19.43; found C: 74.38, H: 5.51, N: 19.37.1,4-di(1H-pyrrol-1-yl)benzene (table 3, entry 10): white solid, mp 234-238℃. 1H-NMR (300MHz, CDCl3): δ= 6.28 (t, 4H), 7.00 (t, 4H), 7.42 (m, 4H). 13C-NMR (75MHz, CDCl3): δ=110.8, 119.3, 121.6, 138.5. Analitically calculated for: (C14H12N2) C: 80.74, H: 5.81, N: 13.45; found C: 80.68, H: 5.69, N: 13.39.
ACKNOWLEDGEMENTS
- Authors thank CSIR, New Delhi for the Junior Research Fellowship to Mr. N. Chandrasekhar.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML