-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2011; 1(2): 81-88
doi:10.5923/j.materials.20110102.13
Hydrotalcite/unsaturated Carboxylic Acid Systems as Coagents in Ethylene-Propylene Copolymer Vulcanization
Magdalena Maciejewska, Alicja Krzywania-Kaliszewska, Ma
Technical University of Lodz, Institute of Polymer and Dye Technology, Lodz, 90-924, Poland
Correspondence to: Magdalena Maciejewska, Technical University of Lodz, Institute of Polymer and Dye Technology, Lodz, 90-924, Poland.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The aim of this work was to study the activity of several hydrotalcite/unsaturated acid systems in the peroxide crosslinking of ethylene-propylene copolymer (EPM). Allylmalonic, citronellic, crotonic, itaconic, sorbic and undecylenic acids, as well as monoallyl maleate, were applied to ensure the activity of hydrotalcite in the peroxide vulcanization of EPM. In this article, we discuss the effect of the obtained coagents with respect to their particle size and tendency to agglomerate, as well as the effect of the applied type of unsaturated acid on the vulcanization kinetics of rubber compounds. The influence of hydrotalcite/unsaturated acid systems on the crosslink density, the mechanical properties of the vulcanizates and their stress relaxation tendency under external deformation were also studied. Hydrotalcite/unsaturated acid systems seem to be active as coagents in the crosslinking of EPM with peroxide. The type of unsaturated acid considerably influences the activity of hydrotalcite toward the ethylene-propylene copolymer. The application of such coagents increases the crosslink density of the vulcanizates and improves their mechanical properties.
Keywords: Hydrotalcite, Coagent, Crosslinking, Ethylene-Propylene Copolymer, Vulcanization
Cite this paper: Magdalena Maciejewska, Alicja Krzywania-Kaliszewska, Ma, Hydrotalcite/unsaturated Carboxylic Acid Systems as Coagents in Ethylene-Propylene Copolymer Vulcanization, American Journal of Materials Science, Vol. 1 No. 2, 2011, pp. 81-88. doi: 10.5923/j.materials.20110102.13.
Article Outline
1. Introduction
- Peroxide vulcanization of polymers is a widely discussed process[1-5]. This process is usually used when good aging resistance, high processing temperature or good compression set at elevated temperature of rubber products is required[6]. However, there are some disadvantages of peroxide crosslinking compared to sulfur vulcanization. Usually, the tensile and tear strength of vulcanizates are reduced in comparison with those of conventionally sulfur-crosslinked samples[1,5]. To overcome this disadvantage and to extend the industrial application of peroxide-crosslinked elastomers, coagents are applied. According to Dluzneski, coagents are multifunctional vinyl monomers that are highly reactive toward the free radicals formed during peroxide vulcanization[2]. Coagents are able to undergo addition and polymerization reactions because their molecules contain double bonds. Because of the high reactivity of coagents toward peroxide radicals, the coagents can be easily grafted to elastomer chains, forming an interpenetrating crosslinked network. Moreover, functional groups of coagents are used to form ionic or complex crosslinks. In this way, coagents are incorporated into the polymer network[4,7]. Coagents can also suppress side reactions that occur during peroxide crosslinking (chain scis-sion and disproportionation) and increase the crosslinking efficiency[7-9]. The most widely used coagents contain allylic, methacrylate, acrylate or maleimide groups[9]. Dikland et al. reported that the application of aliphatic bis(allyl)alkanediols and bis(allyl)poly(ethyleneglycol)s provided high crosslinking efficiencies in the peroxide crosslinking of EPM and reduced the vulcanization time of rubber compounds[4]. It was concluded that coagent bonds were formed as additional crosslinks because of graft polymerization of the allyl moieties of the coagent and covulcanization of coagent domains with the elastomer matrix. De Risi and Noordermeer applied methacrylate coagents to the peroxide curing of ethylene-propylene-diene elastomer and polypropylene blends[3]. The highest crosslinking activity occurred with trimethylol propane trimethacrylate, which also prevented the degradation of polypropylene through chain scission. In recent years, significant interest has been aroused by the application of zinc or magnesium salts of unsaturated carboxylic acids as coagents and fillers for elastomers[10-14]. These salts are formed in situ during the compounding and vulcanization of rubber mixes. Peng et al. reported that in situ prepared zinc dimethacrylate (ZDMA) improved the mechanical properties and crosslink density of ethylene-propylene-diene elastomer[10]. Peroxide initiated crosslinking of the polymer and polymerization of the ZDMA proceeded simultaneously during elastomer vulcanization. The vulcanizates contained both covalent and ionic crosslinks, which arose from the graft polymerization of ZDMA onto the EPDM chains. Similar results were obtained for acrylonitrile-butadiene rubber (NBR)[11], natural rubber[12], and other elastomers reinforced by in situ-formed zinc acrylate and methacrylate[13]. The reinforcement effect of in situ-prepared zinc stearate (ZDS) in NBR was also reported[14]. ZDS was formed by the reaction of zinc oxide with stearic acid during rubber compounding and vulcanization. The presence of ZDS increased the ionic crosslink content of the vulcanizates, their modulus tensile and tear strength. In this work, we employed systems consisting of hydrotalcite and unsaturated carboxylic acids (HTA/UCA) as coagents for the crosslinking of ethylene-propylene copolymer with dicumyl peroxide. This method allowed hydrotalcite to act as the filler and coagent simultaneously. The unsaturated carboxylic acids contained easily-abstractable hydrogen atoms and readily-accessible double bonds that ensured their activity in the crosslinking process. Coagents were obtained during the modification process of hydrotalcite with allylmalonic, citronellic, crotonic, itaconic, sorbic and undecylenic acids, as well as monoallyl maleate. These coagents reacted with the elastomer chains to form covalent bonds. Additionally, ionic crosslinks were formed, thereby leading to an increase in the crosslink density of the vulcanizates and the improvement of their tensile strength. The influence of the HTA/UCA systems on the cure kinetics of rubber compounds and their crosslinking efficiency, vulcanizate crosslink density and tensile strength was discussed.
2. Experimantal Section
2.1. Materials
- Ethylene-propylene copolymer EPM (Dutral CO 034) containing 28% of propylene was obtained from Polimeri Europa. Synthetic hydrotalcite (HTA, Aldrich) with the molecular formula Mg6Al2(CO3)(OH)16 • 4H2O was combined with unsaturated carboxylic acids. Nanosized silica (Aerosil 380, Degussa) was used as an active filler. The characteristics of the unsaturated carboxylic acids (UCA) are presented in Table 1.
2.2. Preparation of Coagents
- Hydrotalcite was mixed with solutions of the unsaturated acids in acetone for 30 minutes during ultrasonic treatment. The mixture was left for 24 hours. Next, the solvent (acetone) was evaporated using a vacuum evaporator at 50 °C. The modified hydrotalcites were dried in a vacuum drier at 60 °C for 96 hours. The quantity of unsaturated acid was 6 g/100 g of hydrotalcite.
2.3. Thermogravimetric Analysis of Coagents
- The coagents were thermally decomposed using a TGA/DSC1 (Mettler Toledo) analyzer. Samples were heated from 25oC to 800oC in an argon atmosphere at a heating rate of 10oC/min.
2.4. Zeta Potential of Coagent Dispersions
- The surface properties of the hydrotalcite after treatment with the unsaturated acids were determined from zeta potential measurements made with a Zetasizer 2000 (Malvern Instruments) apparatus. The zeta potential of the coagent dispersions in water was measured as a function of pH. The concentration of the dispersions was 0.1 g/L, and the pH was adjusted by the addition of an HCl or an NaOH solution.
2.5. Hydrotalcite Aggregate Size
- The size of the hydrotalcite aggregates was determined using a Zetasizer Nano Series S90 (Malvern Instruments) apparatus. The size of the particles was measured by the DLS (Dynamic Light Scattering) method, as applied to suspensions of the particle in water. The concentration of the suspensions was 0.1 g/L.
|
2.6. Preparation and Characterization of Rubber Compounds
- Rubber compounds with the formulations given in Table 2 were prepared using a laboratory two-roll mill. The samples were cured at 160oC until they developed a 90% increase in torque, which was measured by an oscillating disc rheometer. The crosslink density (νT) of the vulcanizates was determined by their equilibrium swelling in toluene based on the Flory-Rehner equation[15]. The Huggins parameter of the elastomer-solvent interaction (χ) was calculated from the equation χ = 0.487 + 0.228Vr (Equation (1))[16], where Vr is the volume fraction of the elastomer in the swollen gel. In order to determine the content of ionic crosslinks in the elastomer network, samples were swollen in toluene in a desiccator with saturated ammonia vapor (25% aqueous solution). The content of ionic crosslinks (Δν) was calculated from Equation (2), where νA is the crosslink density determined for samples treated with ammonia vapor.
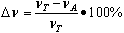 | (2) |
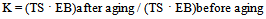 | (3) |
3. Results and Discussion
3.1. Efficiency of Coagent Preparation
- The presence of unsaturated organic acid in the coagents is crucial to ensure their activity in the crosslinking of the ethylene-propylene copolymer with dicumyl peroxide. Therefore, to confirm the presence of organic acids in coagents, thermogravimetric analysis of the modified hydrotalcites was performed. The weight loss of pure hydrotalcite during thermal decomposition and its weight loss after modification with unsaturated acids were compared to determine the presence of unsaturated acid in the HTA/UCA system, and the efficiency of the coagent preparation was calculated. These results are presented in Table 3 and Figure. 1.The various unsaturated carboxylic acids (UCA) exhibited different reactivities toward hydrotalcite. Therefore, the efficiency of the modification of the hydrotalcite surface with UCA differed significantly and was in the range of 30.7 – 59.7%. The highest contents of UCA in 100 g of coagent were achieved for undecylenic and allylmalonic acid, whereas the lowest values were achieved for crotonic and itaconic acid. The UCA content can be expected to affect the activity of the coagent considerably during elastomer crosslinking.
|
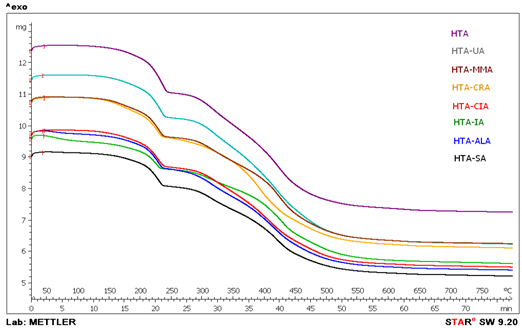 | Figure 1. TGA thermograms of coagents |
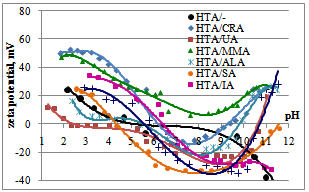 | Figure 2. Zeta potential of coagent dispersions, as a function of pH |
|
3.2. Coagents Particles Size
- The particle size of the coagent is a parameter with a great influence on coagent activity. A reduction of the particle size results in an increase in the coagent specific surface area, thereby providing better contact between its particles and the elastomer chains. The sizes of the coagent particles measured in water dispersion are presented in Table 5.
|
|
3.3. Coagent Dispersion in Elastomer
- SEM images of the vulcanizate surfaces were taken to examine directly the dispersion of coagent particles in the elastomer. These results are presented in Figure. 3 (a-d).
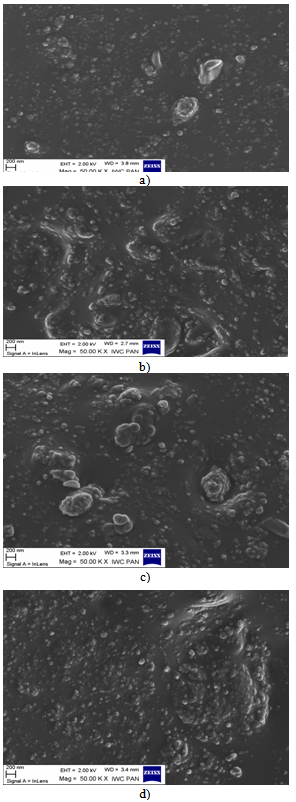 | Figure 3. SEM images of vulcanizates containing: a) HTA/IA, b) HTA/UA, c) HTA/SA, d) HTA/ALA |
3.4. Cure Characteristics and Crosslink Density of Vulcanizates
- The main goal of coagent application is the improvement of the crosslinking efficiency and increased crosslink density of the vulcanizates. We believed that the application of coagents based on hydrotalcite in combination with unsaturated carboxylic acid would result in the formation of additional ionic crosslinks, which should improve the crosslink density of the vulcanizates.To examine the activity of the coagents in crosslinking process, the cure characteristics of the rubber compounds were determined. These results are presented in Table 7.
|
3.5. Mechanical Properties of Vulcanizates
- The influence of coagents on the crosslinking activity of the ethylene-propylene copolymer was estimated from the tensile properties of the vulcanizates. The presence of labile ionic crosslinks in the elastomer network should improve the tensile strength and stress relaxation ability of the vulcanizates. These results are given in Table 8.The results summarized in Table 8 clearly show a high activity of hydrotalcite based coagents in the crosslinking process and a corresponding positive impact on the properties of the vulcanizates.
|
|
3.6. Resistance of Vulcanizates to Weather Aging
- One of the most important properties of saturated elastomers crosslinked with peroxides is their strong aging resistance. Therefore, the effect of coagents on vulcanizate resistance to weather aging was examined through the change in their mechanical properties and crosslink density. We expected that the presence of coagents based on minerals with a layered structure would protect the polymer against the aging process.In Figure. 4, the change in elongation at break upon weather aging is given for vulcanizates containing 7 phr of coagent.
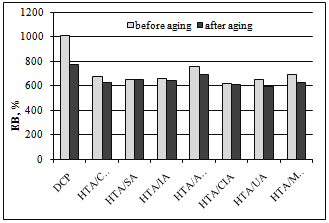 | Figure 4. Vulcanizate elongation at break after weather aging |
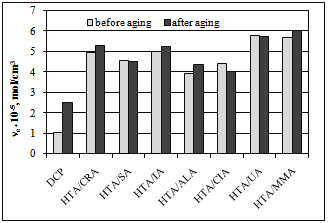 | Figure 5. Vulcanizate crosslink density after weather aging |
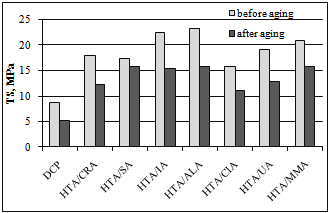 | Figure 6. Vulcanizate tensile strength after weather aging |
|
4. Conclusions
- Hydrotalcite, in combination with unsaturated carboxylic acids, was used as coagent for peroxide-crosslinking of an ethylene-propylene copolymer. The presence of UCA in the coagents was confirmed by the results of zeta potential measurements and thermogravimetric analysis. We conclude that the application of coagents allowed for the realization of vulcanizates with considerably improved mechanical properties and higher crosslink density compared to vulcanizates crosslinked only with peroxide. The crosslink density of EPM vulcanizates was probably improved by the formation of additional ionic crosslinks in the presence of the coagents. The highest ionic crosslink content in the elastomer network was achieved for vulcanizates crosslinked with HTA/CRA and HTA/UA. The ionic crosslink content decreased with the increasing amount of coagent in the elastomer, probably because of the agglomeration of coagent particles in the elastomer, which was confirmed by SEM images. Coagents increased the tensile strength to two or even three times that of the peroxide-crosslinked vulcanizate. The improvement of the tensile strength of the vulcanizates was partially caused by the increase of their stress relaxation ability, as a result of the ionic crosslinks present in the elastomer network.Coagents increased the resistance of vulcanizates to weather aging. Further crosslinking of the elastomers containing coagents during the aging process was greatly reduced in comparison with the purely peroxide-crosslinked vulcanizate. Only a small effect of aging on the crosslink density was observed for vulcanizates with coagents.
ACKNOWLEDGEMENTS
- The authors wish to acknowledge the Polish Ministry of Science and Higher Education and the National Center for Research and Development for supporting this research.
References
| [1] | Henning, S. K., Costin, R., 2006, Fundamentals of curing elastomers with peroxides and coagents, Rubber World, 233, 28-35 |
| [2] | Dluzneski, P. R., 2001, Peroxide vulcanization of elastomers, Rubber Chemistry and Technology, 74, 451-492 |
| [3] | De Risi, F. R., Noordermeer, J. W. M., 2007, Effect of methacrylate co-agents on peroxide cured PP/EPDM thermoplastic vulcanizates, Rubber Chemistry and Technology, 80, 83-99 |
| [4] | Dikland, H. G., Ruardy, T., van der Does, L., Bantjes, A., 1993, New coagents in peroxide vulcanization of EPM, Rubber Chemistry and Technology, 66, 693-711 |
| [5] | Alvarez-Grima, M. M., Talma, A. G., Datta, R. N., Noordermeer, J. W. M., 2006, New concept of co-agents for scorch delay and property improvement in peroxide vulcanization, Rubber Chemistry and Technology, 79, 694-711 |
| [6] | Class, J., 1999, A review of the fundamentals of crosslinking with peroxides, Rubber World, 220, 35-39 |
| [7] | Garcia – Quesada, J. C., Gilbert, M., 2000, Peroxide crosslinking of unplasticized poly(vinyl chloride), Journal of Applied Polymer Science, 77, 2657-2666 |
| [8] | Bucsi, A., Szocs, F., 2000, Kinetics of radical generation in PVC with dibenzoyl peroxide utilizing high-pressure technique, Macromolecular Chemistry and Physics, 201, 435-438 |
| [9] | Dikland, H. G., Hulskotte, R. J. M., 1993, The mechanism of EPDM peroxide vulcanizations in the presence of triallylcyanurate as coagent, Kautschuk und Gummi Kunststoffe, 46, 608-613 |
| [10] | Peng, Z., Liang, X., Zhang, Y., Zhang, Y., 2002, Reinforcement of EPDM by in situ prepared zinc dimethacrylate, Journal of Applied Polymer Science, 84, 1339-1345 |
| [11] | Yuan, X., Peng, Z., Zhang, Y., Zhang, Y., 2000, In situ preparation of zinc salts of unsaturated carboxylic acids to reinforce NBR, Journal of Applied Polymer Science, 77, 2740-2748 |
| [12] | Nie, Y., Huang, G., Qu, L., Zhang, P., Weng, G., Wu, J., 2010, Cure kinetics and morphology of natural rubber reinforced by the in situ polymerization of zinc dimethacrylate, Journal of Applied Polymer Science, 115, 99-106 |
| [13] | Lu, Y., Liu, L., Tian, M., Geng, H., Zhang, L., 2005, Study on mechanical properties of elastomers reinforced by zinc dimethacrylate, European Polymer Journal, 41, 589-598 |
| [14] | Guo, B. C., Chen, F., Chen, W. W., Lei, Y. D., Jia, D. M., 2010, Reinforcement of nitrile rubber by in situ formed zinc disorbate, eXRESS Polymer Letters, 4, 529-538 |
| [15] | Flory, P. J., Rehner, J., 1943, Statistical mechanics of cross-linked polymer networks. II. Swelling, Journal of Chemical Physics, 11, 521-526 |
| [16] | Maciejewska, M., Zaborski M., 2010, Coagents of peroxide crosslinking of elastomers, Przemysl Chemiczny, 89(4), 472-477 |
| [17] | Zecchina, A., Lamberti, C., Bordiga, S., 1998, Surface acidity and basicity: General concepts, Catalysis Today, 41, 169–177 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML