-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Nuclear and Particle Physics
p-ISSN: 2167-6895 e-ISSN: 2167-6909
2016; 6(4): 80-87
doi:10.5923/j.jnpp.20160604.02

Effect of Grain Size on the Radon Exhalation Rate and Emanation Coefficient of Soil, Phosphate and Building Material Samples
S. Harb, N. K. Ahmed, Sahar Elnobi
ERML, Physics Department, Faculty of Science, Qena, South Valley University, Egypt
Correspondence to: S. Harb, ERML, Physics Department, Faculty of Science, Qena, South Valley University, Egypt.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Our present work was focused on the effect of grain size on the emanation coefficient, mass and area exhalation rate in different grain size of the soil, phosphate and building material samples. These samples were fractionated into seven-grain sizes each (2000-1800, 1800-1000, 1000-560, 560-425, 425-200, 200-80 and < 80 μm) and employing AlphaGUARD to determine the radon exhalation rate in these samples. The data analyses were performed to determine emanation coefficient, mass and area exhalation rate. Results revealed an inverse relationship between radon exhalation and grain size in soil and building materials (sand, granite and marble) samples while the trend is not clear in the phosphate and limestone samples. The emanation coefficient of Rn-222 was constant in different grain sizes of all samples.
Keywords: AlphaGUARD, Exhalation, Grain Size, Emanation, Radon and Building Materials
Cite this paper: S. Harb, N. K. Ahmed, Sahar Elnobi, Effect of Grain Size on the Radon Exhalation Rate and Emanation Coefficient of Soil, Phosphate and Building Material Samples, Journal of Nuclear and Particle Physics, Vol. 6 No. 4, 2016, pp. 80-87. doi: 10.5923/j.jnpp.20160604.02.
Article Outline
1. Introduction
- Radon is an odourless, colourless, inert gas with isotopes of relatively short half-lives, there are no chemical or mass spectrometric techniques with sufficient sensitivity to measure it. Consequently, it must be determined using techniques that depend on the radioactive properties either of radon itself or its decay products. The radon gas is created as the first disintegration product of 226Ra. There is three radon (Rn) isotopes naturally present in the environment: 222Rn, a member of the uranium decay series, 220Rn, a member of the thorium decay series, and 219Rn, a member of the actinium decay series. 220Rn and 219Rn are often referred to as thoron and actinon, respectively (Ishimori et al., 2013) [1].Radon is highly radioactive and decays by the emission of alpha particles. Every nucleus of 222Rn eventually decays through the sequence Po-218, Pb-214, Bi-214, Po-214 and Pb-210. With each transformation along this path the nucleus emits characteristic radiations: alpha, beta particles, gamma rays, or combinations of these. A particular radon nucleus may decay at any time, but it is most likely to decay between now and eight days. 222Rn is the most important in radiological protection because of its relatively long half-life of 3.82 days. The migration of radon gas occurs the form of diffusion and advection. Radon gas arrives the indoors from different sources, such as soil or rock under or surrounding the buildings, building materials, water supplies, natural gas and outdoor air. (Akerblom and Wilson, 1981; Colle′ et al., 1981; Durrani and Ilic, 1997) [2-4]. The major source of Radon in the atmosphere at least 80% is from emanations from soil that derived from rocks. (Deepanjan, 2000; Badr et al., 1996; Abumurad et al., 2001; Khan et al., 1987; Jonsson, 1987) [5-9]. These rocks contain some uranium, where the decay of 238U through 226Ra gives radon to which people are exposed. Certain types of rock, including granites, dark shale, light-colored volcanic rocks, sedimentary rocks containing phosphate and metamorphic rocks derived from these rocks have higher average uranium contents (Virk et al., 1993) [10]. A soil radon activity unnatural environmental condition is affected by soil moisture content, Air pressure variations and temperature and structure of soil. Freed sandy soil lets the maximum diffusion of radon gas, whereas frozen, compressed or clay soil inhibits its flow. Eventually, it exhales into the atmosphere. Phosphate rocks contain relatively high concentrations of naturally occurring radioactive materials from the uranium and thorium decay series (238U and 232Th) (UNSCEAR, 1993)) [11]. With increasing the progress in phosphate industry in Egypt from year to year, manufacturing of phosphate fertilizers and using phosphate fertilizers containing uranium are ways in which the workers, public and the environment are exposed to enhanced doses of radiations (UNSCEAR, 1993, IAEA, 2004, Othman, 2007)) [11-13]. During handling, packing and transporting of fertilizers some workers can receive additional external gamma exposure at dose rates up to 0.8 µGy/h (Steinhäusler, 1984)) [14]. Phosphate industry is producing dust with high intensity and with different grain size and it can be a negative impact on the respiratory tract in industrial workers. When they exposed for a long time can cause an inflammation. Buildings are very important in human life because people spend most of their time (about 80%) indoors (home or office). Where sand, granite, marble and limestone sample as building materials are very common in Egypt. Therefor constructions Workers are exposed to the high intensity of dust (small particle sizes). So these samples have been selected to be under investigation study.The exhalation is radon atoms that have been transported to the ground surface and then exhaled to the atmosphere (Ishimori et al., 2013) [1]. Radon exhalation depends mainly on 226Ra content and mineral grain size, its transport in the earth governed by geophysical and geochemical parameters while exhalation is controlled by hydrometeorological conditions (Etiope and Martinelli, 2002) [15]. Grain size is one of the important factors that control radon concentration and exhalation rate in soil. The effect of the grain size factor has been studied (e.g. Markkanen and Arvela, 1992; Shweikani et al., 1995; Menetrez and Mosley, 1996; Sundal et al., 2004; Barillon et al., 2005) [16-20]. They determine how much radium is near enough the grain surface to allow radon to escape into pore spaces. The measurement of indoor radon exhalation rates are of utmost importance because inhalation of radon and its progeny contributes more than 50% of the total radiation dose from the natural sources. By separating the radon exhalation rate by either the area of the exhaling surfaces or by the mass of the sample, the area (radon flux Bq/ m2h) and mass radon exhalation rates (Bq/kgh) can be calculated.The alpha decay of 226Ra has enough energy to propel the progeny 222Rn atom out of the mineral lattice (with a range of 34 nm in quartz, given in Sakoda et al. (2011) [21]) and into the pore space between the mineral grains (Tanner, 1980) [22]. This process of 222Rn escaping into the pore space is called emanation, the fraction of radon atoms generated that escape the solid phase in which they are formed and become free to migrate through the bulk medium is called the emanation coefficient (Ishimori et al., 2013 [1]). Pore and grain size and the distribution of 226Ra and 238U in the grain affect the emanation coefficient (Sakoda et al., 2011) [21].The present study illustrates the measurements of emanation coefficient and radon exhalation from phosphate, soil, and building materials (sand, granite, marble and limestone) samples using active techniques and study of the effect of grain size on the mass and area exhalation rate in different grain size of the samples.
2. Materials and Methods
2.1. Sampling and Samples Preparation
- Six kinds of materials were under investigation. These materials collected from different locations in Aswan and Qena governorate, Egypt as shown in Fig. 1 Phosphate sample obtained from the El-Sabaea phosphate factory (25° 11’ N, 32° 39’ E) with different grain size. Soil, sand and limestone samples were collected from Hagaza village in Qus city. Granite and marble samples were collected from Aswan governorate.
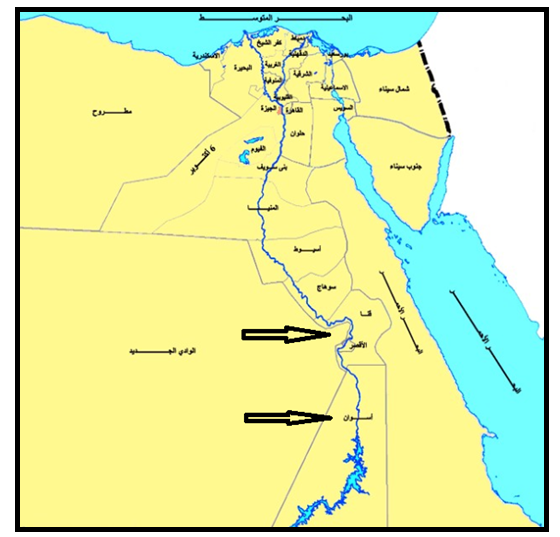 | Figure 1. Sampling location |
2.2. Measuring Systems
2.2.1. AlphaGUARD
2.2.1.1. Radon Exhalation Rate
- Ionization chamber AlphaGUARD PQ2000PRO along with the additional special equipment AquaKIT [23] was used for determining 222Rn exhalation activity concentration in phosphate, soil and building materials samples. The active setup used for radon exhalation measurements of samples with AlphaGUARD is shown in Fig. 2. At equilibrium state, the final activity of exhaled radon inside that vessel is given by:
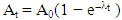 | (1) |
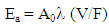 | (2) |
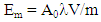 | (3) |
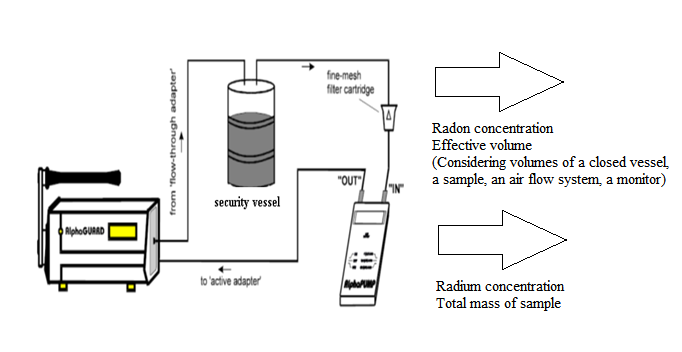 | Figure 2. Use of AlphaPUMP in a closed gas cycle for measuring set-up (AquaKIT, 1999) |
2.2.1.2. Emanation Coefficient of Radon-222
- The total activity of radon released into the air from the sample material is evaluated by a measurement of radon concentration and the effective volume of the measurement system Fig. 2. The emanation coefficient is calculated by (Ishimori et al., 2013) [1]:E = VC/MRWhere E is the emanation coefficient, V is the effective volume of the sampling device (m3), C is the radon concentration (Bq/m3), M is the total mass of the sample (kg) and R is the radium activity concentration (Bq/kg). The effective volume is determined by considering the volume of the closed vessel, of all material inside the closed vessel, of the loop system for radon measurement and the radon monitor. The total activity of radium in the sample material can be determined by alpha spectrometry (IAEA, 2010) [27].
3. Results and Discussions
3.1. Radon Exhalation
- The calculated values of radium content and radon exhalation rate in different grain sizes from 80 to 2000 μm of the phosphate, soil and building materials (sand, granite, marble and limestone) samples with the help Alpha GUARD has been presented in Table 1. It can be seen from the results that the radon exhalation rate of phosphate is high, where the radium is high in phosphate rock (Negm, 2009) [28]. The radon concentration of different grain size of the phosphate sample varies appreciably from one-grain size to another due to the variation of radium content as shown in Fig. 3.Radium content of different grain sizes of phosphate sample ranged from 108.35±8 to 914.15±65 Bq/kg with a mean value of 260.52±19 Bq/kg. The mass exhalation rate falls within the range of 0.82±0.06 to 6.91±0.49 Bq/kgh with a mean value of 1.97±0.14 49 Bq/kgh. The area exhalation rate falls within the range of 14.49±0.23 to 122.28±1.33 Bq/m2h with a mean value of 34.85±0.44 Bq/m2h. From Table 1, the highest radium content, mass and area exhalation rate of phosphate sample were found to be 914.15±65 Bq/kg, 6.91±0.49 49 Bq/kgh and 122.28±1.33 Bq/m2h, respectively in grain size >560 μm and <1000 μm of the phosphate sample. While, the lowest radium content, mass and area exhalation rate were found to be 108.36±8 Bq/kg, 0.82±0.06 49 Bq/kgh and 14.49±0.23 Bq/m2h, respectively in the grain size >425 μm and <560 μm of the sample. The results indicate that the radon exhalation rates strongly depend on the grain size of phosphate sample (Rashmi, 2009; Breitner, 2010) [29, 30].For soil sample Radium content falls within the range of 264.54±19 to 355.65±25 Bq/kg with a mean value of 300.68±21 Bq/kg, mass exhalation rate ranged from 2±0.14 to 2.69±0.19 Bq/kgh with a mean value of 2.27±0.16 Bq/kgh. Area exhalation rate falls within the range of 35.38±0.45 to 47.57±0.57 Bq/m2h with a mean value of 40.22±0.5 Bq/m2h. In soil sample, the highest radium content and exhalation rate of radon in fine grains of 425-200, 200-80 and <80 μm. While, the particle sizes of 2000-1800, 1800-1000, 1000-560 and 560-425 μm show lower radium content and exhalation rate of radon as shown in Fig. 4. An inverse correlation between grain size and exhalation rates can be observed. It may be due to the decrease in pore space between grains due to which more wide space becomes available for radon to exhale from the surface (Tuccimei, 2006; Breitner, 2010) [31, 30].
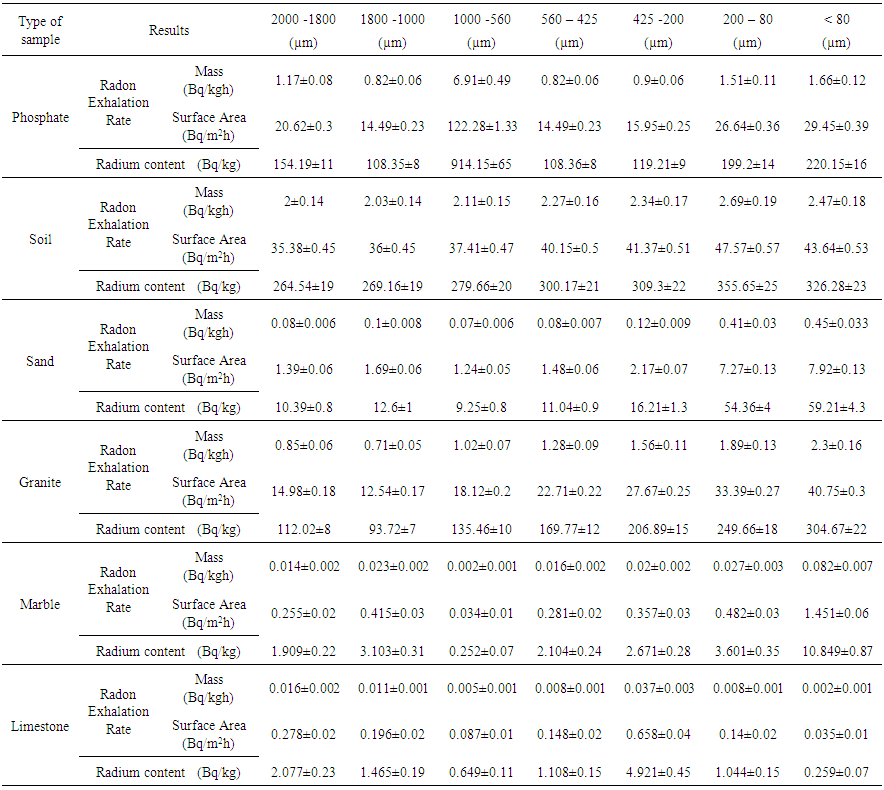 | Table 1. Radon exhalation rate and radium content in phosphate, soil, and building materials (sand, granite, marble, and limestone) samples and in their different grain sizes (µm) using Alpha GUARD |
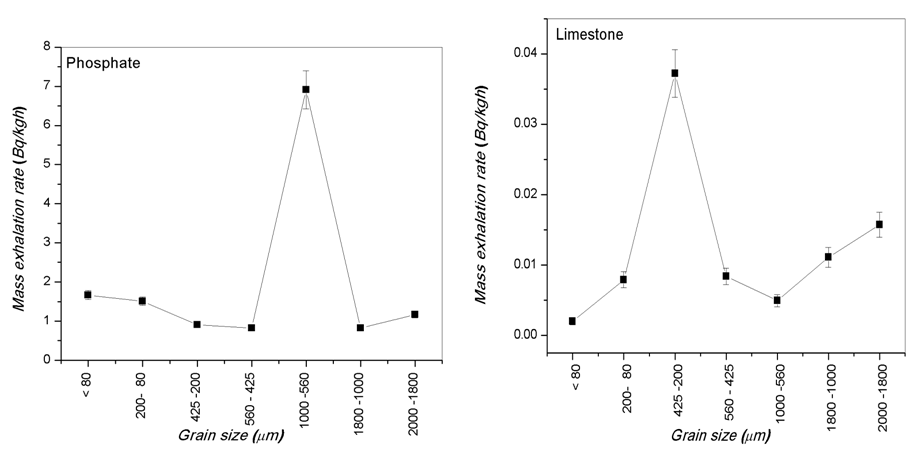 | Figure 3. Relationship between mass exhalation rare and grain size in different grain sizes of phosphate and limestone samples |
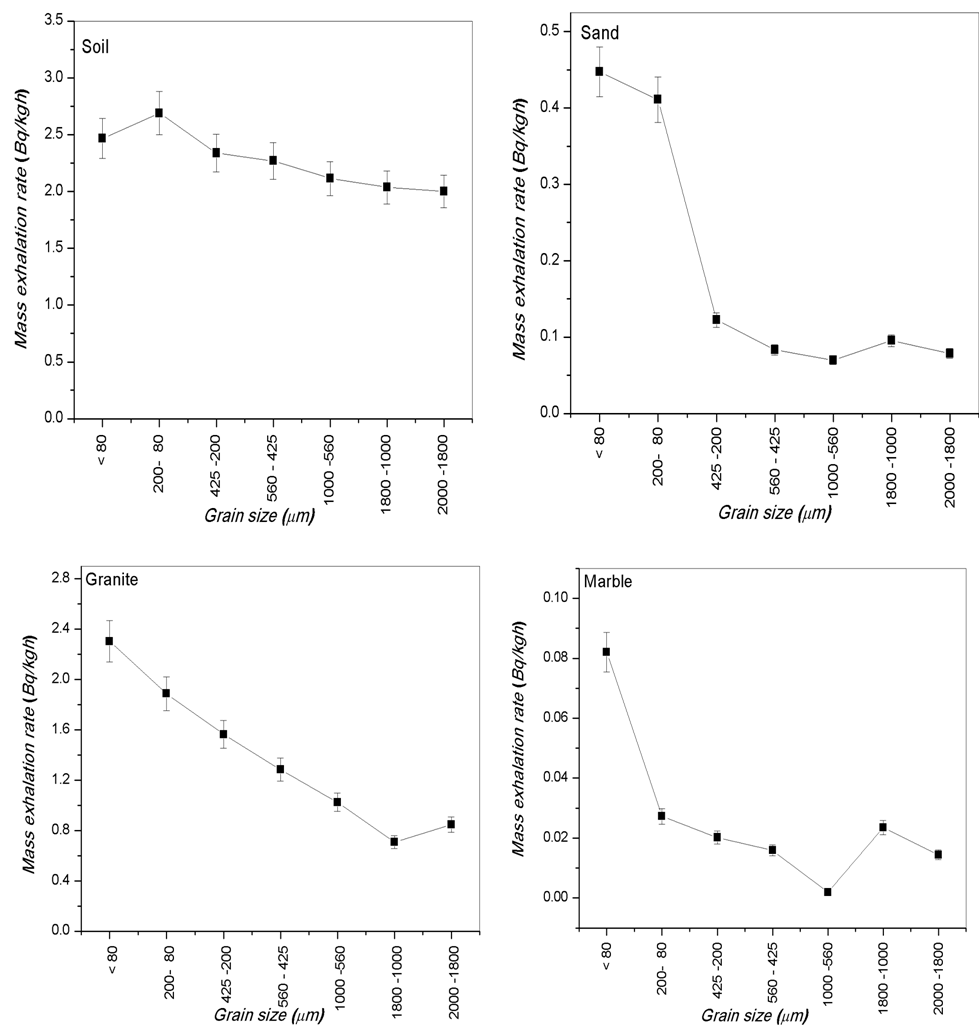 | Figure 4. Relationship between mass exhalation rare and grain size in different grain sizes of soil, sand, granite and marble samples |
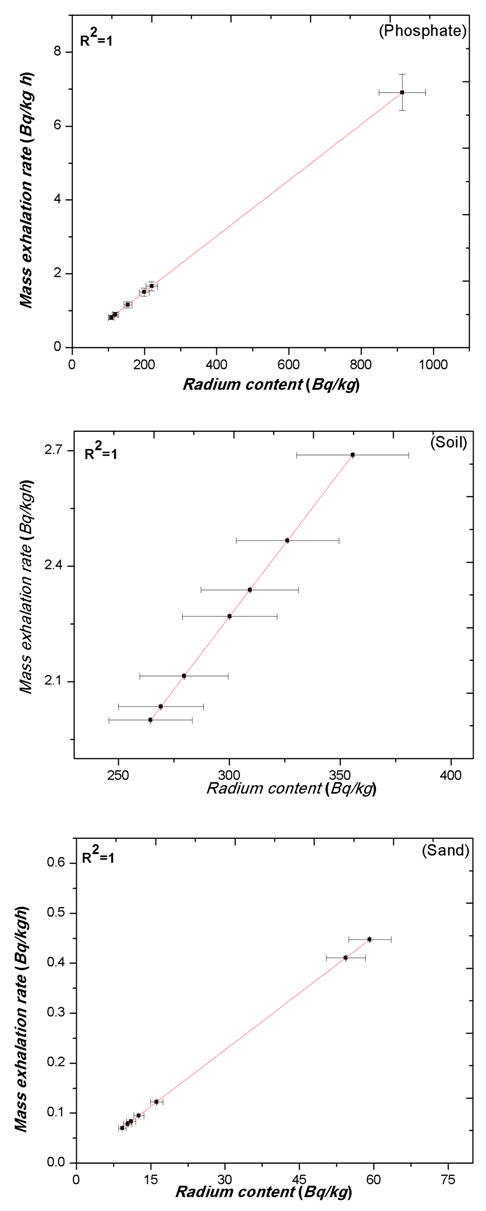 | Figure 5. The relation between the Radium content (Bq/kg) & mass exhalation rate (Bq/kgh), of Rn-222 in different grain sizes of phosphate, soil and sand samples |
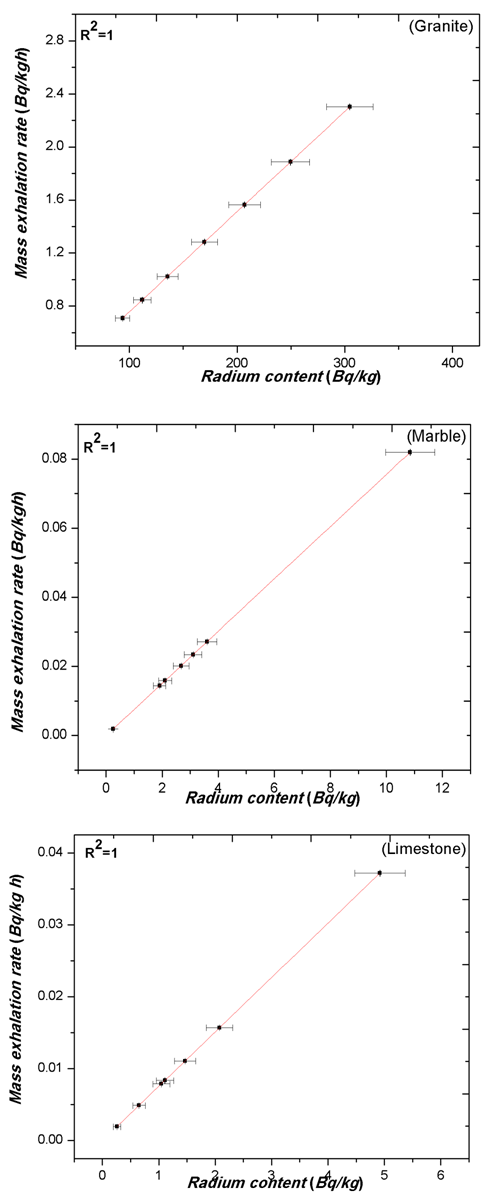 | Figure 6. The relation between the Radium content (Bq/kg) & mass exhalation rate (Bq/kgh) of Rn-222 in different grain sizes of granite, marble and limestone samples |
3.2. Emanation Coefficient
- The emanation coefficient was constant regardless of the particle diameter in all samples because of radium were mainly distributed on the grain surface. The particle size and shape determine in part how much uranium and radium is close enough to the surface of the grain to allow the radon to escape into the interstitial pores (Ishimori et al., 2013) [1].
4. Conclusions
- The results indicate that the measured values of radon exhalation rates strongly depend on the grain size of the samples. The radium content and exhalation rate of all samples increase with decreasing the grain size except phosphate and limestone samples, where this trend is not clear. This trend may be due to the reduction in pore space between grains due to which more wide space becomes available for radon to exhale from the surface. The results indicate that phosphate generally has higher exhalation rate than other building materials and soil. While granite sample shows a possible dependency of exhalation rate on grain size and other samples show a very small variation of exhalation rate. Higher radiation levels of granite sample are associated with igneous rocks. The obtained values of radon exhalation rate for all samples, except phosphate sample, are well below the world average value of 57.60 Bq/m2h. Strong positive correlation has been observed between radium content, area exhalation rate and mass exhalation rate. The emanation coefficient was constant regardless of the particle diameter in all samples.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML