Eletta B. E.
Department of Applied Science, Kaduna Polytechnic, Kaduna, PMB 2021, Nigeria
Correspondence to: Eletta B. E. , Department of Applied Science, Kaduna Polytechnic, Kaduna, PMB 2021, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
The method of Atomic Absorption Spectroscopy has been employed to analyze three heavy elements; Cu, Fe and Mn in cored samples from the underlying sediments from Ahmadu Bello University Water Dam. Additionally, the variations observed for Fe and Mn further showed the capability of the AAS technique to distinguish small difference in concentrations of these elements in samples collected from nearly the same environment. By the results presented in this work for Mn, Fe and Cu, the chemical baseline data have been further enriched for these elements in the A.B.U Water Dam.
Keywords:
Trace Element, Atomic Absorption Spectroscopy, Heavy Metals, Sediments, Pollution, Water Dam
1. Introduction
Heavy metals are considered as a group of major pollutants of marine ecosystem, particularly those elements which are toxic to marine organisms and human beings of which As, Cd, Fe, Cr, Ni, Hg, Se, Zn, Cu and Mn are generally held to be most important.In the assessment of the pollution situation of these elements in the source of water supply to Ahmadu Bello University Community, in Zaria, Nigeria, knowledge of their present levels in the sediment and suspended matter (sludge) of River Kubanni is necessary. Besides, the analysis of heavy metals in the sediments underlying the A.B.U Water Dam is important as it will help enrich the baseline data for the area under study. Three of heavy metals, i.e. Cu, Mn, and Fe, whose cathode lamps are readily available, due to the researchers’ funding limitation, were eventually analyzed in 34 sediment and 3 sludge samples.Atomic absorption spectroscopy requires the following two essential conditions:a) A source of electromagnetic radiation of the resonance frequency i.e. light which can be absorbed by atoms in the ground state.b) A population of ground state atoms able to absorb the light of the correct resonance frequency.When a magnetic light wave of energy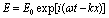 | (1) |
Where ω = 2πv = angular frequencyK = 2π/λ = wave numbertravels through an absorbing sample with absorption coefficient α and length L, the intensity I0 of the incident wave decreases to: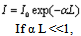 | (2) |
The relative attenuation can be expressed as 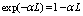 Therefore
Therefore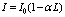 hence
hence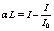 | (3) |
In order to measure α, one has to measure a small difference of two large quantities, which becomes less and less accurate with decreasing α L.In AAS, lines are classified to their integrated coefficients.Absorbance A is defined as: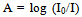 | (4) |
At a time temperature T (in K), the ratio of the number of atoms Nu in an excited (upper) state ‘a’ to the number of atoms Ni in the ground state is given by the Maxwell-Boltzman expression;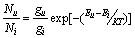 | (5) |
Where Eu and Ei are the energies of the excited and ground states respectively.gu and gi are the statistical weights of the respective states.K is the Boltzman’s constant.Putting equation (9) in equation (12) we have;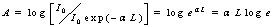
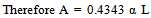 | (6) |
This implies that the abundance is directly proportional to the absorption coefficient and therefore to the concentration of the analyte.Without dilution;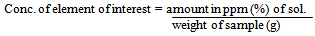
2. Methodology
Samples were collected during the dry season by making use of plastic cylindrical covers. This was done by squeezing the cover to sediments under water until the desired depth was achieved. This was done at different sample points across the dam labelled appropriately.The sediments collected were then sectioned into plastic bags according to depths and taken to the laboratory where the samples were kept in the oven at 600C for one week until the samples were completely dried. Sludges were also collected from the base of the sedimentation tank from the water treatment section of the dam. The sludges were collected into plastic bags and kept in the oven alongside the sediments to ensure dryness.Dried samples were then homogenized by grinding using the Agate Mortar and pestle. Homogeneity of the samples was further ensured by making each sample to undergo traditional quartering technique by making each sample to go through several sub-divisions. The grinded samples were then kept in sealed bottles awaiting analysis procedures. The grinding was done until fine powder of ca 150 – 200 mesh was obtained.1.0 gramme of each sample were weighted into PTEE (Polytetrafluoroethylene) beakers and moistened with distilled water.In this study, interest is not based on silicon determination hence the method of dissolution is one based on Hydrofluoric acid addition with the help of which silicon can be lost from the matrix.It is of advantage if silicon is to be removed during the acid attack procedure such advantages include the following:(i)Resultant solutions are far more stable. Silica bearing solutions tend to hydrolyze and precipitate on standing for long period.(ii) Silicon and fluorine cause interference with some photometric determinations.(iii) The corrosive action of HF is removed, permitting manipulations with normal laboratory glass ware.(iv)Such procedures reduce the salt content of resultant solutions. This is important as the method of atomic absorption spectroscopy is being used for determinations. 5ml of Nitric acid was then added to the sample in the PTEE beaker after which 10ml of Hydrofluoric acid was then added. The resulting solution was then placed on a sand bath at about 600C in a vacuum cupboard until dryness was achieved. In some cases, fresh additions of the reagents were necessary for complete loss of silica.The reason for the addition of distilled water and Nitric acid is to soften the matrix and to reduce a likely vigorous reaction between the HF and the sample. The dried sample were then recovered from the vacuum cupboard and made into solution by adding concentrated Hydrochloric acid and distilled water 1:1 (v/v). It was ensured that all samples were taken up into solution and kept in labelled plastic bottled and ready for analysis.
2.1. Preparation of Standard Solution for AAS
A single element standard solution was prepared for each element of interest. This was done by dissolving the high-grade metal or appropriate compound of the element in a small volume of conc. HNO3 acid. About 1mg/ml (1000ppm) each of Cu, Mn and Fe standard solution was prepared as stocks from which subsequent dilutions suiting the instrument calibration ranges for the elements of interest were made.The standard used as check for the analysis using AAS is the International Atomic Energy Agency’s (IAEA) Lake Sediment (SL). The digestion and dissolution was also done for this standard as was done for the samples. The concentration of elements in the IAEA SL- standard is shown in Table 3.
3. Results
AAS Analysis was done for three elements; copper, manganese and Iron. The table below shows the concentration ranges and the corresponding wavelength for each element being analysed. | Table 1.Conc. Range and Wavelength for Mn, Fe and Cu |
| | Element | Range in (ppm) | Wavelength Å | | Mn | 1.5 – 3.0 | 2795 | | Fe | 2.5 – 5.0 | 2483 | | Cu | 2.5 – 5.0 | 3244 |
|
|
4. Discussion
The result for the concentration ranges of Copper, Iron and Manganese in the sediments, sludge and in the Standard Reference Material (SL-1) are presented in Tables 2 and 3. From the tables, one observes that the concentrations for Cu, Fe and Mn in both the sludge and underlying sediments are less than those for the Standard Reference Material (SL) which has been prepared to represent sediments exhibiting normal concentrations for elements in the list.There is therefore no obvious pollution by Cu, Fe and Mn in the sediment. In other words, the Cu, Fe and Mn contents belong to the natural background values.3-D plots for the distribution for the three elements are displayed in Figures 1, 2 and 3. The result for the distribution of Iron in A.B.U Water Dam is shown in Table 2 and the 3-D plots for the distribution is shown in Figure 1 From the plot, it is observed that the highest concentration of Iron is along the North-Western end of the dam. This may be due to accumulation of more waste taking place along that side of the dam.The result for the distribution of Manganese in A.B.U Water Dam is shown in Table 2 and the 3-D plot for the distribution is shown in Figure 2. From this interesting plot, the concentration of Manganese is observed to be higher at the source end of the Dam which could be due to the sudden slowing down in the speed of the water thereby causing it to deposit most of its contents at the entry point.From figure 3, it is observed that the concentration of Copper across the dam is almost constant except for very few troughs or variations. | Table 2. Distribution of Copper at various Sample Points |
| | Sample | Depth (cm) | Conc. of Cu (ppm) | Conc. of Fe | Conc. of Mn | | A1 | 2.0 | 10.0 | 36.5 | 110 | | A2 | 4.0 | 10.0 | 38.0 | 130 | | A3 | 6.0 | 10.0 | 24.2 | 100 | | B1 | 2.0 | 10.0 | 9.5 | 110 | | B2 | 4.0 | 10.0 | 29.5 | 90 | | B3 | 6.0 | 10.0 | 12.2 | 130 | | C1 | 2.0 | 0.0 | 15.5 | 70 | | C2 | 4.0 | 10.0 | 8.5 | 60 | | C3 | 6.0 | 10.0 | 14.8 | 90 | | D1 | 2.0 | 10.0 | 11.0 | 80 | | D2 | 4.0 | 10.0 | 26.0 | 60 | | D3 | 6.0 | 10.0 | 35.0 | 60 | | E1 | 2.0 | 10.0 | 27.5 | 120 | | E2 | 4.0 | 10.0 | 24.9 | 60 | | F1 | 2.0 | 0.0 | 635.0 | 80 | | F2 | 4.0 | 0.0 | 224.0 | 170 | | G1 | 2.0 | 10.0 | 179.0 | 60 | | G2 | 4.0 | 10.0 | 603.5 | 50 | | G3 | 6.0 | 0.0 | 23375.0 | 60 | | H1 | 2.0 | 10.0 | 430.5 | 90 | | H2 | 4.0 | 10.0 | 4890.0 | 80 | | H3 | 6.0 | 10.0 | 777.5 | 70 | | H4 | 8.0 | 10.0 | 3375.0 | 60 | | I1 | 2.0 | 10.0 | 61.5 | 90 | | I2 | 4.0 | 10.0 | 37.5 | 80 | | I3 | 6.0 | 10.0 | 109.5 | 100 | | J1 | 2.0 | 10.0 | - | 170 | | J2 | 4.0 | 10.0 | 54.5 | 80 | | K1 | 2.0 | 10.0 | 91.5 | 160 | | K2 | 4.0 | 10.0 | 64.5 | 180 | | K3 | 6.0 | 10.0 | 55.0 | 150 | | L1 | 2.0 | 10.0 | 60.5 | 150 | | L2 | 4.0 | 10.0 | 102.0 | 170 | | L3 | 6.0 | 10.0 | 99.0 | 100 |
|
|
| Table 3. Sludge and Lake sediment concentration for the elements |
| | Cu(ppm) | Fe(ppm) | Mn | | S1 | 20 | 5.0 | 130 | | S2 | 10 | 0.0 | 110 | | S3 | 20 | - | - | | SS1 | 10 | 530 | - | | SS2 | 675 | - | - | | SLI – 1 | 0.0 | 375 | 4750 | | SLI – 2 | 0.0 | - | 2750 | | SLI – 3 | - | - | 3000 | | SLI – 4 | 0.0 | 760 | 4000 | | SLI – 5 | 0.0 | 440 | 3000 | | SLI – 6 | 0.0 | 430 | 3000 | | SLI - 7 | 0.0 | 740 | 3250 |
|
|
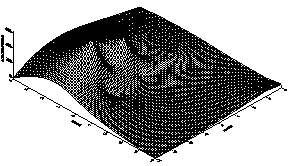 | Figure 1. 3D plot of the Fe Distribution in ABU Water Dam |
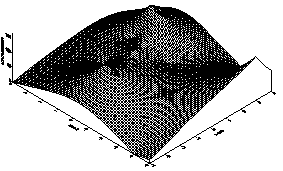 | Figure 2. 3D plot of the Mn Distribution in ABU Water Dam |
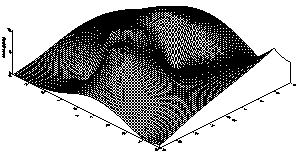 | Figure 3. 3D plot of the Cu Distribution in ABU Water Dam |
5. Conclusions
The overall conclusion that could be drawn from this work is that for the three elements Copper, Iron and Manganese which were analysed in the underlying sediment, there seems to be no serious indication of pollution arising from their accumulation over the period of this study.
References
| [1] | Claude, V. (1976): Optical Atomic Spectroscopic Methods Trace Analysis; Spectroscopic Methods for elements. WINEFORDNER, J.D. (Editor). Chemical Analysis Vol. 46, John Wiley (Pub). |
| [2] | Doherty, V.F., Kanife, U.C., Ladipo, M.K. and Akinfemi, A. (2011). Heavy Metal Levels in Vegetables from selected markets in Lagos, Nigeria. Electronic Journal of Environmental, Agricultural and Food Chemistry. Vol 10(2) pp 1887-1891. |
| [3] | Ewa, I.O.B, Dim, L.A., Oladipo, M.O.A. (1992): Cluster Analysis of elemental concentration of cored Nigerian river sediments. Journal of Environmental Science and Health, part A Vol. A27, Number 1, Deker (pub.). |
| [4] | Lawson, E.O. (2011). Physico-Chemical parameters and Heavy metal content of water from the mangrove swamps of Lagos Lagoon, Lagos, Nigeria. Advances in Biological Research. Vol. 5(1), pp. 8 – 21. |
| [5] | Mustapha, A.O. (1991): Characterization of the moderated Neutron field around on Isotopic Am-241/Be source. Unpublished M.Sc. Physics Thesis. |
| [6] | Nworgu, O.D. and Osahon, O.D. (2011) . Determination of the distribution patterns and abundances of Trace Elements in Nigerian Crude Oil using the AAS Technique. International Journal of Engineering Research in Africa. Vol. 5(64), pp. 64 – 73. |
| [7] | Oladipo, M.O.A (1988): Trace element analysis of Corinthian Pottery and Related clays, Ph.D Thesis. Thesis Abstract International B, Vol.37(4), 1988. Microfish Ref. No.: 37-7276. |
| [8] | Parsons, M.L. (1976):Nuclear Methods. Trace Analysis: SpectroscopicMethods for elements. WINEFORDNER, J.D. (Editor), Chemical Analysis, Vol.46, John Wiley (Pub.) |
| [9] | Samali, A., Kirim, R.A. and Mustapha, K.B. (2011). Qualitative and Quantitative evaluation of some herbal teas commonly consumed in Nigeria. African Journal of Pharmacy and Pharmacology. Vol.6(6), pp. 384 – 388. |
| [10] | Skibniewski, M.; Kosla, T.; Skibniewska, E.M.; Kupczynka, M.; Makowiecka, M.; Klimkowska, P.; Urbanska-Slomka, G. (2006). Copper and Magnesium content in the Hair of dogs and cats in the living warsaw area. Journal of Environmental studies.vol 26 Issue 2A, p168. |
| [11] | Tijani, N., Ike, P.O., Usman, B.B., Malami, D.I. and Matholo, A. (2012). Trace Elemental Analysis of Nigerian Petroleum Product using AAS Method. International Journal of Scientific and Engineering Research vol 3 Issue 2. |
| [12] | Yebra-Biurrun, M.C. Cespon-Romro, R.M. (2007). Fast ultrasound-asssted extraction of copper, Iron, Manganese and zinc from human hair samples prior to flow injection flame atomic absorption spectrometric detection. Analytical and Bioanalytical Chemistry; vol 388 issue 3 p711. |

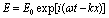
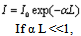
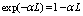 Therefore
Therefore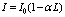 hence
hence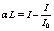
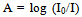
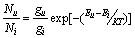
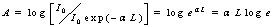
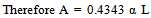
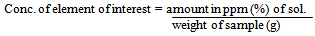



 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML