-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Mechanical Engineering and Automation
p-ISSN: 2163-2405 e-ISSN: 2163-2413
2016; 6(2): 25-29
doi:10.5923/j.jmea.20160602.01

Oscillatory-Type DNA Amplification System with 8051 Based Temperature Controller
Jyh Jian Chen 1, Wei Hua Chen 2, Hsu Jeng Liu 2
1Department of Biomechatronics Engineering, National Pingtung University of Science and Technology, Taiwan
2Department of Mechanical Engineering, National Pingtung University of Science and Technology, Taiwan
Correspondence to: Jyh Jian Chen , Department of Biomechatronics Engineering, National Pingtung University of Science and Technology, Taiwan.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this work an oscillatory-type DNA amplification system is designed. The high temperature zone for denaturation is set at the center of the thermal cycling system, and the low temperature annealing zone using the thermoelectric cooler can effectively speed up the cooling rate. With utilizing the 8051 microprocessor to integrate the temperature controller, the cost and the volume of the thermal control system can be greatly reduced. The temperature difference between the surface and the central part of the chamber is determined in this study. The effects of various setting temperatures and moving speeds of the chamber at different isothermal regions are demonstrated. The thermal isolation by the air gaps between the heater blocks is obvious. Besides, the increasing of the moving speeds causes the decreasing of the residence time, and the heat transfer between the chamber and the ambient material is not sufficient. It can be found that the required PCR temperature is not obtained.
Keywords: DNA amplification, Microprocessor, PCR, Thermal isolation
Cite this paper: Jyh Jian Chen , Wei Hua Chen , Hsu Jeng Liu , Oscillatory-Type DNA Amplification System with 8051 Based Temperature Controller, Journal of Mechanical Engineering and Automation, Vol. 6 No. 2, 2016, pp. 25-29. doi: 10.5923/j.jmea.20160602.01.
Article Outline
1. Background/ Objectives and Goals
- Since polymerase chain reaction (PCR) has been invented in the early 1980s by Saiki et al. (1985), PCR has become one of the most significant methods for the identification and replication of DNA strands. The PCR process consists of three steps. (1) Denaturation: one double-stranded DNA is separated into two single strands at high temperature (about 95°C), (2) Annealing: primers bind onto their complementary site of the single-stranded DNA at low temperature (about 55°C), and (3) Extension: these DNA strands are extended by thermostable DNA polymerase at intermediate temperature (about 72°C). Nowadays the PCR is mostly executed in a thermal cycler. The amplification efficiency is not only related to the sample pre-preparation, but also the temperature of the sample as the sample runs through the thermal cycles. In order to complete the PCR process accurately, a precise heat transfer module is necessary.The manipulation approaches of the PCR system can be categorized into the chamber type and the continuous-flow type. The chamber-type device is a miniaturized version of the commercial thermal cycler. The DNA mixture is kept stationary, and the temperature of the reaction chamber is cycled. Nothrup et al. (1993) presented the first microfabicated PCR chip. This chamber-type chip contained a reaction chamber with an integrated heating system. For the continuous-flow PCR (CFPCR) chip, the reaction mixture moves through three different temperature zones in a thin channel or a tube to complete the PCR. Kopp et al. (1998) presented the first CFPCR chip. The chip was put on three copper isothermal blocks. Compared with a typical chamber-type PCR, CFPCR has the benefit of very rapid thermal cycling. The residence time in which a sample is exposed to each temperature zone as well as the number of thermal cycles for CFPCR are determined by the arrangement of the heating zones rather than by the heating and cooling processes as required by the chamber-type PCR.There are three main types of the CFPCR reactors: unidirectional, closed-loop, and oscillatory reactors. Each one has its own characteristic advantages (Chen et al., 2013). To miniaturize the volume of the PCR system, the temperature control systems with a microprocessor were utilized over the last ten years. Tsai and Sue (2006) integrated a temperature control system of PCR with a microprocessor and the associated control circuits. Wang et al. (2009) utilized the microcontroller to modulate the temperature of each PCR chamber. Han et al. (2014) fabricated a real-time temperature monitoring and control system based on the microprocessing unit for online temperature control. Angus et al. (2015) designed a temperature control module with an Arduino Uno microprocessor.In recent years, many researchers were interested in the molecular analysis system at the point of care. And portable devices are becoming an essential part of the point of care testing. The PCR device integrated with the microprocessor can reduce the system volume and provide product results quickly. In this study, we design an oscillatory-type DNA amplification system with 8051 based temperature controller. The system combines with the merits of the chamber-type PCR and the CFPCR devices. The samples are placed in a fixed chamber. Three isothermal zones are established in the system. The samples are oscillated and contacted with three different isothermal zones to complete the thermal cyclers.
2. Methods
- The oscillatory-type DNA amplification system was depicted schematically and demonstrated in our previous work (Chen et al., 2011). It comprises a reaction chamber, three aluminum blocks with individual heating modules, some thermocouples for sensing the temperatures and a moving stage with a linear motor. The PCR chamber is designed and fabricated using a CNC milling machine (EGX400, Roland, USA) with aluminum material. The position of the reaction chamber is controlled by a linear motor (SmartT VL-ST60, Montrol, Taiwan). The reaction chamber moves through three sequential temperature zones. One PCR thermal cycle is completed when the reaction chamber leaves the extension zone. After a designated number of thermal cycles, the reagent can be taken out of the chamber for further analysis. In this work the denaturation region is set at the middle part of the system to speed the PCR.The thermal control systems are utilized to control the power input into the heater and the thermoelectric (TE) cooler and create a stable temperature distribution within the aluminum block. The diagram and the photograph of the control system were shown in our latest study (Chen et al., 2015). The temperature control system consists of an AT89S51 microprocessor, an amplifier, a D/C converter, a seven-segment display, a key-press input, an interface with serial port (RS-232) and a power output. The 8051 microcontroller receives feedback signals from a DS-1821 thermal sensor. All programming is written in the C++ language. The DS-1821 sensor is used to monitor the temperatures on the aluminum surfaces and provide the feedback signals for a proportional-integral-derivative (PID) controller. Measurements from the sensors are output to the seven-segment display and through an RS-232 serial port to a personal computer. The circuit layout is shown in Fig. 1.
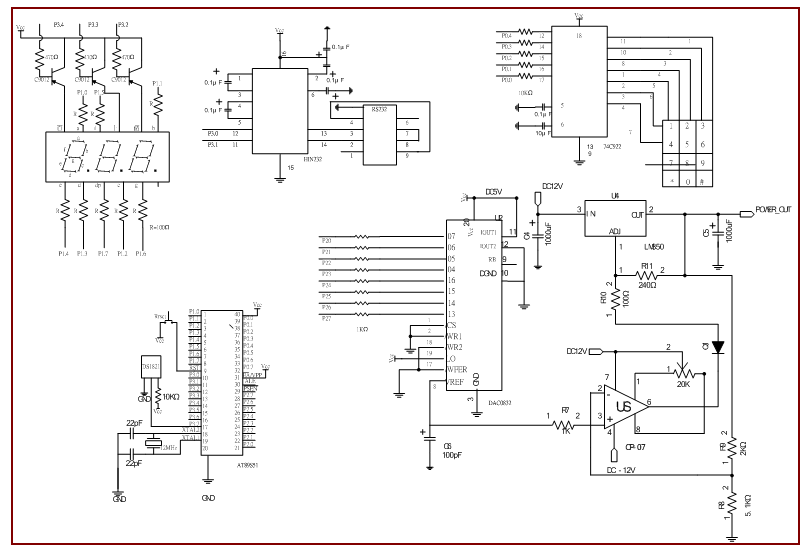 | Figure 1. The circuit layout of the thermal control system |
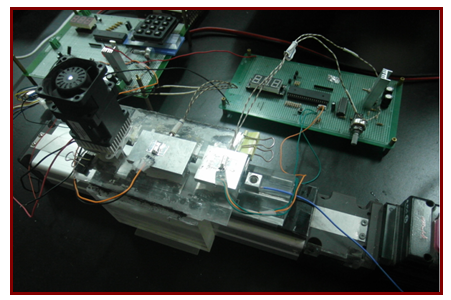 | Figure 2. The oscillatory-type DNA amplification system with 8051 based temperature controller |
3. Results
- In the following we show the heat transfer in a DNA solution inside a PCR chamber exposed to three isothermal heating zones and two convective cooling zones. This study presents the effects of the operational parameters on the chamber temperature.In order to perform a comprehensive analysis of the heat transfer mechanism in solution and chamber, the temperature profiles inside the chamber and on the chamber surface are utilized to demonstrate the thermal characteristics of the system and shown in Fig. 3. One thermocouple is inserted into the water inside the chamber volume and near the center of the chamber to measure the temperature of the central location of the chamber. And the other one is inserted into a small hole on the chamber wall to monitor the temperature of the aluminum chamber. During PCR, the DNA mixture inside the chamber experiences the thermal cycling process and it is impossible to monitor the mixture temperature. However, the measured surface temperatures of the reaction chamber are usually utilized to make sure that the PCR setting temperatures are reached. The required PCR temperatures can be obtained by measuring the surface temperature of the chamber and then adding the temperature difference between the surface and the PCR mixture. From Fig. 3 it shows the temperature difference between the temperature profiles of the chamber surface (blue line) and the chamber center (red line). Inside the thermal regions for denaturation and extension, the temperature inside the chamber is higher than that on the chamber surface. And the temperature inside the chamber is lower than that on the chamber surface inside the annealing region. The repeatability and the stability of the oscillatory system are also shown in Fig. 3. It demonstrates that the temperature curves of the chamber for 6 times are similar.
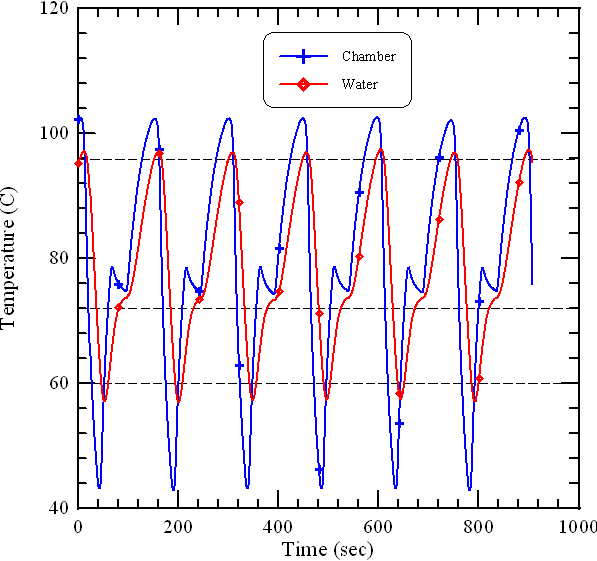 | Figure 3. The temperature profiles of the chamber center (red line) and the chamber surface |
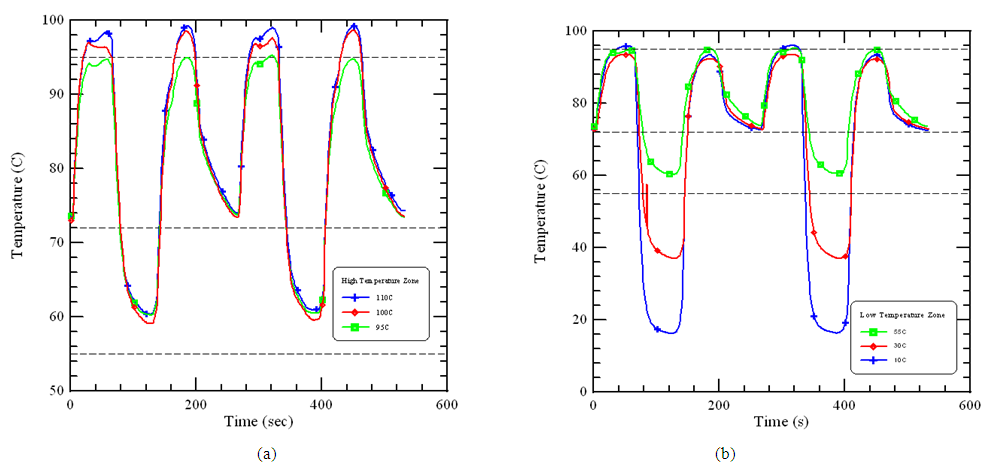 | Figure 4. The central temperature profiles of the sample at different temperatures of (a) high temperature zone and (b) low temperature zone |
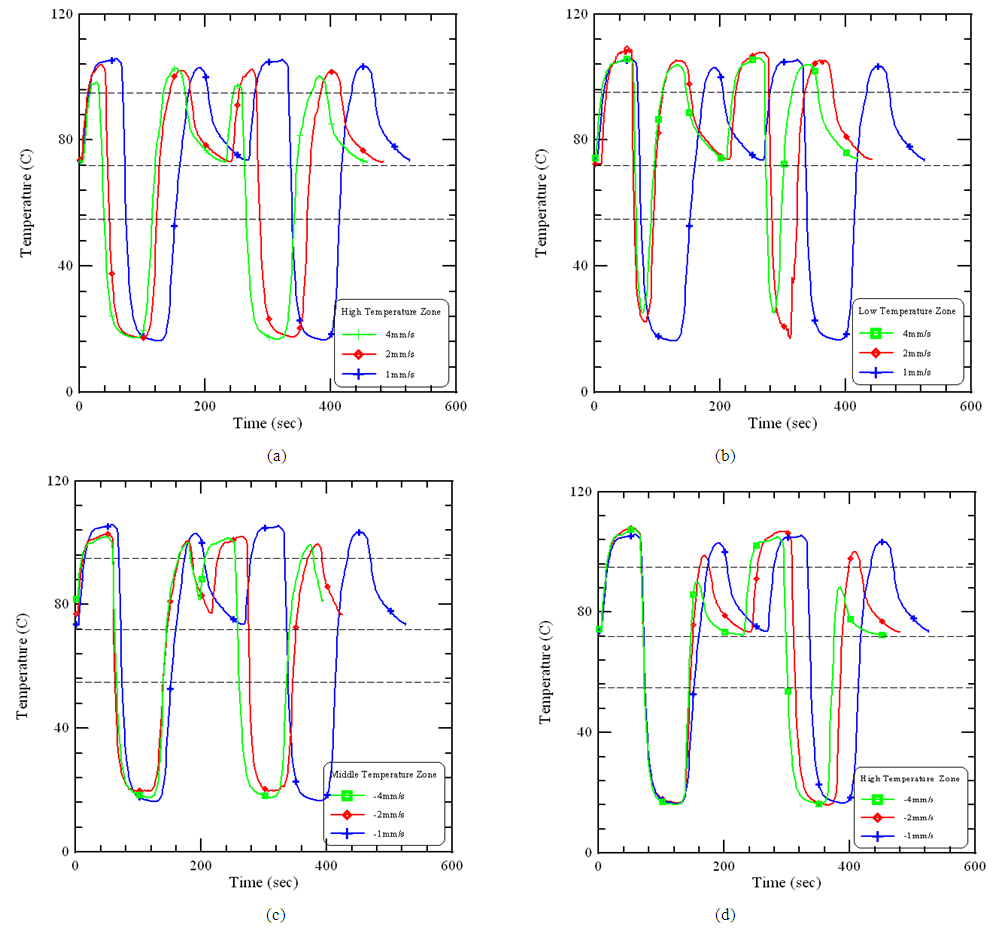 | Figure 5. The experimental temperatures of the cases at various forward speeds or backward speeds at different isothermal regions |
4. Conclusions
- In order to reduce the reaction time during thermal cycles and the volume of the system, an oscillatory-type DNA amplification system is modified from our previous design. The thermal control module is integrated by utilizing the 8051 microprocessor and the associated electric citcuits. The sample is oscillated and comes in contact with three different isothermal zones to complete thermal cycles. The central and surface temperatures of the chamber with various moving speeds are measured. The thermal isolation by the air gaps of 12 mm between the heater blocks is obvious. Finally, it is found that the required PCR temperature is not obtained as the moving speeds are higher than 1 mm/s. This work should be interesting to persons involved in the designs of a thermocycler and the PCR processes in the bioreaction chamber.
ACKNOWLEDGEMENTS
- The authors would like to thank the National Science Council of the Republic of China for financially supporting this research under Contract No. MOST 104-2313-B-020-001- and MOST 104-2627-B-020-001-.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML