-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Mechanical Engineering and Automation
p-ISSN: 2163-2405 e-ISSN: 2163-2413
2015; 5(2): 67-71
doi:10.5923/j.jmea.20150502.01
Kinetic Study of the Removal of Residual Copper(II) on Activated Carbon and Alternative Adsorbents
Thallis M. Souza1, Adelir A. Saczk1, 2, Zuy M. Magriotis1, 2, Priscila F. de Sales1, Ricardo F. Resende1, Felipe M. Pinto1, Bianca M. C. Botrel1
1Departamento de Química, Universidade Federal de Lavras, Lavras, Brasil
2Laboratório de Gestão de Resíduos Químicos, Diretoria de Meio Ambiente, Universidade Federal de Lavras, Lavras, Brasil
Correspondence to: Thallis M. Souza, Departamento de Química, Universidade Federal de Lavras, Lavras, Brasil.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The objective of this study was to adjust the kinetic data of the adsorption of residual copper(II) to the pseudo-first-order model, pseudo-second-order model, intraparticle diffusion and Elovich. The waste contained copper(II), generated in the analysis of protein determination at Departamento de Ciência dos Alimentos, of Universidade Federal de Lavras, Brazil. The concentration of copper(II) was 11.39 mg L-1, determined by atomic absorption spectrometry, and the value found for pH was 13. Adsorption was studied employing kaolinite, advanced oxidation process (AOP) sludge and cotton lint as alternative adsorbents. Results of the percentage removal of copper(II) were compared with adsorption onto commercial activated carbon. The adsorption process was conducted at room temperature (25 ± 1°C), using 0.10 g of adsorbent and 10.0 mL of waste. The percentage removal of residual copper(II) obtained for the adsorbents active carbon, kaolinite, AOP sludge and cotton lint was 95.1, 94.5, 77.2 and 82.1%, respectively. The results showed that kaolinite was the most adequate alternative adsorbent. In this case, the final concentration of copper(II) was lower than the allowed by the Brazilian law for effluent discharge, which is 1.0 mg L-1. The adjusted results showed that the model proposed by Elovich was more adequate for removal onto active carbon and cotton lint while, for adsorption onto kaolinite and AOP sludge, the most adequate was the pseudo-second-order model.
Keywords: Adsorption, Alternative adsorbents, Chemical waste, Copper, Kinetic models
Cite this paper: Thallis M. Souza, Adelir A. Saczk, Zuy M. Magriotis, Priscila F. de Sales, Ricardo F. Resende, Felipe M. Pinto, Bianca M. C. Botrel, Kinetic Study of the Removal of Residual Copper(II) on Activated Carbon and Alternative Adsorbents, Journal of Mechanical Engineering and Automation, Vol. 5 No. 2, 2015, pp. 67-71. doi: 10.5923/j.jmea.20150502.01.
Article Outline
1. Introduction
- Heavy metals are substances considered persistent in the environment, making them a major cause of pollution, since they are not appropriately discarded [1].Copper is an important element in industry (galvanizing processes, wood treatment and conservation, production of textile dyes, synthetic dyes and metal alloys [2]; in agriculture (fungicides [3]), besides being a micronutrient present in metalloproteins [4] and aiding in the metabolism of carbohydrates and nitrogenous compounds in vegetables [5].However, copper can cause two diseases, as a result of genetic mutations: Menkes disease is responsible for neurologic complications, and can lead to death, especially in childhood, and Wilson's disease, which results in the toxic accumulation of copper in the liver and, in some cases, in the brain [6]. In addition, the improper application of pesticides containing heavy metals and organic compounds with a high contamination capacity into the biota, as well as the disposal of untreated waste, have resulted in the pollution of soil and water bodies [7].According to the World Health Organization (WHO), the maximum concentration of copper for drinking water is 1.0 mg L-1. At higher concentrations, copper makes water inadequate for consumption, since it causes an unpleasant taste, in addition to effects to the organism. In Brazil, the National Council for the Environment (Conselho Nacional de Meio Ambiente, CONAMA) states, in its resolution nº. 430 of 13 May 2011, that the maximum concentration for the discharge of effluents for dissolved copper is 1.0 mg L-1 [8].In order to meet the requirements of the law, so that there is no damage to the environment and to any living organism, several processes in the removal of metal ions have been studied, thus minimizing the generation of contaminants in effluents containing such substances. Among the most widespread processes, adsorption, which has been an efficient process in the treatment of effluents, stands out [9].Alternative adsorbents, such as kaolinite, which is a clay mineral [10], are abundant in nature, in addition to by-products of agricultural or industrial processes. These materials have a high efficiency in the removal of various contaminants, besides having a low cost; therefore, they are promising adsorbents, capable of replacing commercial activated carbon, making the effluent treatment process less costly [11].
2. Material and Methods
2.1. Adsorbate
- The waste generated by the analyses of protein content performed in the Department of Food Science of Universidade Federal de Lavras (UFLA) was used as an adsorbate, and it was collected by the Chemical Waste Management Laboratory (LGRQ). The residue, which had pH 13, consisted of CuSO4, K2SO4, H2SO4 and NaOH.
2.2. Adsorbents
- Natural kaolinite was provided by Mineradora Química e Minérios (Ijaci, MG, Brazil) and did not undergo any previous treatment, being sieved in the fraction corresponding to 0.425 mm.Cotton lint was a residue from research activities performed by the Seed Pathology Laboratory, in the Department of Forestry of UFLA. Once emulsified in sulfuric acid, it was first washed with distilled water to reduce the acidity of the starting material. The residue was then left at rest and subjected to drying. The sample was subsequently vacuum filtered until the pH of the last wash was kept at around 7, followed by drying in an oven at 70°C for 24 hours. Cotton lint was previously sieved in a fraction corresponding to 0.425 mm, in order to be used as an adsorbent material.AOP sludge was obtained from the degradation, via photo-Fenton reaction, of organic waste collected by the LGRQ. AOP sludge was sun-dried, sieved in a fraction corresponding to 0.425 mm, and was subsequently used as an adsorbent.For comparison purposes, the adsorption test was carried out with powdered activated carbon (Cromoline, Brazil).
2.3. Adsorption Kinetics
- Adsorption experiments were conducted in batch at room temperature (25 ± 1°C) in a shaking table at 120 rpm. 10-mL aliquots of the residue were placed in contact with 0.100 ± 0.005 g of the adsorbents in a kinetics monitored between 0 and 360 minutes. Samples removed at defined time intervals were filtered, and the remaining concentrations were determined by flame atomic absorption spectrometry (FAAS), in an AA 110 Varian Spectra equipment, at a wavelength of 325 nm.The percentage removal of copper(II) was calculated according to Equation 1:
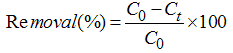 | (1) |
3. Results and Discussion
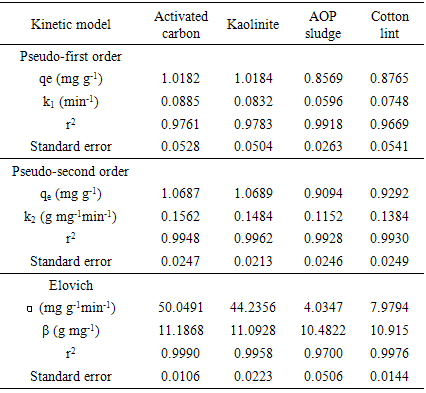
- One of the most important parameters for the evaluation of adsorption efficiency is the kinetic, which studies the time-evolution of a physical phenomenon.The kinetic models that were used to evaluate the efficiency of the adsorbent in metal removal and to predict the mechanisms of adsorption process by pseudo-first-order, pseudo-second-order and Elovich.The pseudo-first-order kinetic model described by Lagergren (1898) [12] is often used to understand the adsorption mechanism of adsorbates in liquid phase, and is represented by Equation 2:
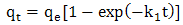 | (2) |
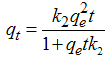 | (3) |
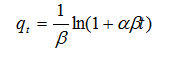 | (4) |
|
|
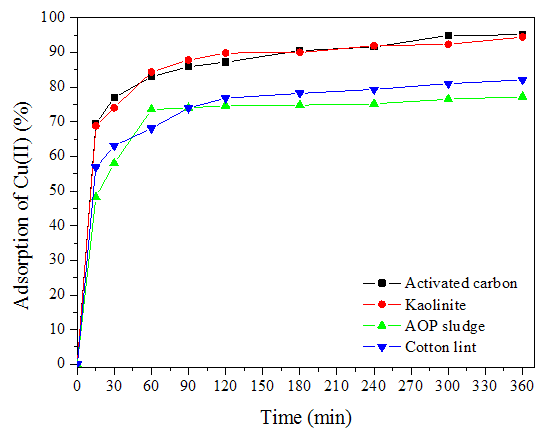 | Figure 1. Adsorption kinetics of residual copper(II) |
4. Conclusions
- The results for the adjustments of the data to the adsorption of residual copper(II) showed that the kinetic profiles followed distinct models, allowing to infer that the removal process involved surfaces with different characteristics. While the model proposed by Elovich was the most suitable for the removal on activated carbon and cotton lint, adsorption on kaolinite and POA sludge followed the pseudo-second-order model.Kaolinite was the alternative adsorbent with the highest efficiency in copper(II) removal, which was 94.5%, similar to the commercial activated carbon (95.1%).Additionally, the remaining concentration of copper(II) in the process in which kaolinite was used as an adsorbent was below the maximum limit specified by the Brazilian law. Therefore, this material presented a high potential to replace activated carbon in the treatment of copper(II) waste, generated in protein analyses.
ACKNOWLEDGEMENTS
- The authors would like to thank Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Financiadora de Estudos e Projetos (FINEP), Pró-reitoria de Planejamento e Gestão (PROPLAG/UFLA) for the financial support and grants provided, and the Chemistry Department/UFLA for the FAAS analyses.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML