-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2025; 13(2): 29-36
doi:10.5923/j.jlce.20251302.01
Received: Aug. 12, 2025; Accepted: Sep. 6, 2025; Published: Sep. 20, 2025

Green Chemistry for High School Students: Synthesis of Biodiesel from Waste Cooking Oil
Omar A. El Seoud1, Maria Helena Zambelli2, Raquel N. Guimarães3, Kátia Regina V. Roa4, Francisco Mateus Alves5, Lize Silvério6, Elisa H. I. Hediger7, Melissa K. C. Ufer7, Naved I. Malek8, Nathália F. Peres1
1Institute of Chemistry, University of São Paulo, São Paulo, Brazil
2Humboldt College, São Paulo, Brazil
3Prof. Luiz Gonzaga Righini State High School, São Paulo, Brazil
4Prof. Mario M. D. de Aquino State High School, São Paulo, Brazil
5Major Telmo Coelho Filho State High School, São Paulo, Brazil
6Immaculate Mary College, São Paulo, Brazil
7The Swiss-Brazilian School, São Paulo, Brazil
8S. V. National Institute of Technology, Surat, Gujarat, India
Correspondence to: Omar A. El Seoud, Institute of Chemistry, University of São Paulo, São Paulo, Brazil.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The project reported here is for senior high school (HS) students. It is coordinated by the Institute of Chemistry (Chem-USP) and is linked to the University of São Paulo sustainability program. The objective of the project is to introduce HS students to green chemistry using a “hands-on” approach. Biodiesel (BD) was synthesized by transesterification of waste cooking oil (WCO) with bioethanol (EtOH) and the produced BD was evaluated as a fuel for diesel engines. The relevance of the subject is that Brazil is a major producer of sugarcane-based EtOH and vegetable oils that are used for obtaining BD. By employing WCO, we introduced the students to the concept of recycling. The project is composed of 3 stages: (1) Explanation and a questionnaire about biofuels at the HSs, before visiting Chem-USP; (2) One-day visit to Chem-USP to analyze WCO; synthesize BD, and being introduced to gas chromatography; (3) Presentation by the students at the HSs of the results obtained, and evaluation of the project. Students, 116, from 6 HSs located in the metropolitan São Paulo area participated in this project. To synthesize BD, they used WCO from refined palm oil (RPO), EtOH, and H2SO4 as a transesterification catalyst. Their visits to Chem-USP were from 9 to 16 h, with a time-out for lunch. During the experiment, they were supervised by teaching assistants (TAs). In addition to BD synthesis, the students determined the acid and saponification values of RPO and WCO and were introduced to gas chromatography. After their visits, the TAs determined the viscosity of the products (mixtures of BD + unreacted WCO) and sent the results to the HSs, along with a reference (viscosity x BD%) curve for calculation of BD yield. The latter curve is that for mixtures of (authentic BD + WCO) that we constructed at Chem-USP. The students presented their data in class and evaluated the project. The pre-visit evaluation showed their interest in biofuels and recycling, whereas the post-visit counterpart showed that they appreciated this “hand-on” approach to recycling as a part of sustainability.
Keywords: Biodiesel Synthesis, Education for Sustainable Development, High School-University program
Cite this paper: Omar A. El Seoud, Maria Helena Zambelli, Raquel N. Guimarães, Kátia Regina V. Roa, Francisco Mateus Alves, Lize Silvério, Elisa H. I. Hediger, Melissa K. C. Ufer, Naved I. Malek, Nathália F. Peres, Green Chemistry for High School Students: Synthesis of Biodiesel from Waste Cooking Oil, Journal of Laboratory Chemical Education, Vol. 13 No. 2, 2025, pp. 29-36. doi: 10.5923/j.jlce.20251302.01.
Article Outline
1. Introduction
- The 30th United Nations Climate Change Conference (COP30), will be held in Belém, Brazil, in November 2025. It will prioritize sustainability goals through various initiatives, with emphasis on renewable energy, forest preservation, education and public awareness [1]. Diesel engines have been serving as workhorse in several fields, including transportation; crop harvesting; power generation and mining. Over the last century, numerous improvements were introduced to make these engines more efficient, more powerful, and less polluting. Even with the advent of battery-operated vehicles, the diesel engine will still be in use for years, thanks to the stringent emission rules introduced and increased engine efficiency [2]. The use of mixtures of (petroleum-based) diesel oil and biodiesel (BD) is contributing to the continued use of the diesel engine. This is because BD is a more efficient fuel than diesel oil, as indicated by the corresponding cetane numbers (CN), 55-65 and 40-45, respectively, and the fact that BD is essentially sulfur-free [3]. Compared to diesel oil, the use of BD from vegetable oils and fats results in the reduction of greenhouse gas emissions by 40% to 69% [4]. For obvious reasons (human consumption; cost) there is an increased interest in substituting edible oils, e.g., those from soybean, canola, peanuts and sunflower as raw materials for BD production with non-edible oils [5], and waste cooking-oil (WCO) [6]. The annual world production of the latter oil is ca. 190 million metric tons [7]. With this background, we introduced a project for senior high school (HS) students on the transformation of WCO into BD. The theme of this project is relevant because Brazil is a major producer of vegetable oils, in particular soybean oil, [8] and is the second sugarcane-based bioethanol (EtOH) producer, worldwide [9]. Additionally, the use of refined, i.e., bleached and deodorized oil from the mesocarp part (pulp) of the palm fruit (RPO) for food frying is popular in the north and northeast parts of Brazil. In addition to the recycling aspect, the project addresses the following question: what happens (chemically) to the oil when used for frying (at ca. 180°C) [10] in the presence of the water released from food? The project is composed of 3 stages: (1) Explanation and a questionnaire about biofuels at the HSs, before visiting the Institute of Chemistry of the University of São Paulo (Chem-USP). These biofuels include BD and EtOH. Note that the use of EtOH as fuel is not new to these students because it has been employed in Brazil as a car fuel, either alone or mixed with gasoline, since 1975 [11]; (2) One-day visit to Chem-USP to analyze RPO and WCO; carry out the BD synthesis experiment and being introduced to gas chromatographic analysis. The oil analysis includes determination of the acid value (AV) and saponification value (SV) by acid-base titration, and demonstrations of potentiometric titration and gas chromatography; (3) Presentation by the students at the HSs of the results obtained. As shown by the students´ final evaluation, the project was successful thanks to its objective, namely, solving an environmental problem by recycling. The students appreciated their introduction to new techniques, including potentiometric titration and gas chromatography.
2. Experimental
2.1. Materials
- Commercial absolute ethanol (EtOH) 99.8%; ethylene glycol (EG); H2SO4 (98%); standardized HCl, NaOH and KOH solutions were from LabSynth, São Paulo, and were used as received. RPO (Tauá food company) was purchased at a local supermarket. The WCO was obtained from the university’s central restaurant. It was treated with 5% (w/v) anhydrous MgSO4, and then clarified by centrifugation at 7000g, using Sorvall Legend X1R centrifuge. The material needed for the experiments includes Eppendorf-type pipettes of the appropriate volumes; three-necked round-bottom reaction flasks; Erlenmeyer flasks; semi-analytical balance; hotplates with magnetic stirring; glass cylindrical oil baths containing ethylene glycol (EG); red spirit-filled thermometers, separation funnels, and Falcon types.
2.2. Procedures
2.2.1. Quantitative Transformation of Refined Palm Oil and Waste Cooking Oil into the Corresponding Biodiesel
- This experiment was carried out by the TAs before the visits to Chem-USP. RPO and WCO were quantitatively transformed into the corresponding BDs by reacting each oil with a large excess of EtOH (alcohol: oil volume ratio = 30:1), using concentrated H2SO4 as a catalyst (catalyst/ethanolic oil solution = 10%, w/w) [12]. The completeness of the reaction was confirmed by CG/MS analysis using Shimadzu model CG msQP2010, equipped with BPX5 (5% phenyl methylpolysiloxane) - 30m capillary column, that was heated according to the following program: 5 min at 120°C; increase to 240°C at 8°C/minute; 8 min at 240°C. These samples will be denoted BD-100%-RPO and BD-100%-WCO, respectively.
2.2.2. Determination of the Acid Values of Refined Palm Oil and Waste Cooking Oil
- The students determined the free fatty acids present in each oil by acid-base titration using phenolphthalein as an indicator. Subsequently, they calculated the acid values (AV) of both samples, according to Equation 1, as given elsewhere [13].
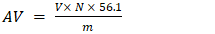 | (1) |
2.2.3. Determination of the Saponification Values (SVs) of Refined Palm Oil and Waste Cooking Oil
- Due to lack of time, the students received these oils already saponified by using alcoholic KOH, as given elsewhere [15]. They back titrated the excess KOH present with a standardized HCl solution using phenolphthalein indicator. Subsequently, they calculated SV (given in mg KOH/g oil) by using Equation 2:
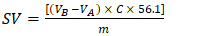 | (2) |
2.2.4. Determination of the Iodine Value (IV) of Waste Cooking Oil
- The IV is the mass of iodine, in g, consumed by 100 g of WCO. It was determined by a green method using aqueous ethanol solvent [16], by adding the I2 solution to the oil, followed by titration of the excess halogen using a standardized Na2S2O3 solution. This experiment was carried out by the TAs as a demonstration, using Orion Star T920 Redox Titrator with platinum electrodes. The value of IV is calculated by Equation 3:
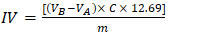 | (3) |
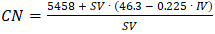 | (4) |
2.2.5. Synthesis of Biodiesel
- This experiment was carried out by the students in groups, each of 2 or 3. The reaction conditions and the order of the experiments were generated by the TAs, using version 13.3 of the Statistica software (StatSoft, TIBCO Software).The instructions given to the students were as follows: Place the provided EG heating bath (containing a magnetic bar) on the top of a hotplate/magnetic stirrer. Place the (hotplate + EG-bath) on a laboratory scissor jack. Insert the provided thermometer into the bath and begin the (stirring + heating) of the EG until its temperature reaches 100 ± 5°C. Concurrently, introduce (do not drop) a magnetic stirring bar into a 3-necked, 125 mL round-bottom flask; mark the flask with the name of the school and the group (e.g., Humboldt-G1, etc). Use the graduated cylinder provided to measure 20 mL of the WCO. Using an addition funnel, introduce the oil into the reaction flask. Using a burette, introduce the appropriate volume of EtOH. Once the oil and EtOH were introduced, close the three "necks" of the round-bottom flask with the provided polypropylene stoppers. Assemble the transesterification reactor above (not inside) the EG-bath, by inserting a reflux condenser provided with a CaCl2 drying tube in the central “neck” of the flask. When the temperature of the EG bath reaches the indicated range (100 ± 5°C), circulate water into the reflux condenser. Ask the instructor to introduce the H2SO4 catalyst (caution!), then use the laboratory jack to slowly raise the hot plate/EG-bath (caution!) until the liquid in the reaction flask is slightly below the level of EG of the heating bath. When EtOH begins to boil, start recording the reaction time.At the end of the specified time (see Table 1), turn off the heating, carefully lower the hot plate/EG-bath (caution!), place the reaction flask into a cold-water bath, wait for the reaction mixture to cool down, and then carefully disassemble the equipment. Use the provided Eppendorf-type pipette to introduce the volume of ethanolic NaOH solution indicated in Table 1 to neutralize the acid catalyst. Next, use an addition funnel to transfer the reaction mixture into a 125 mL separatory funnel (caution: the funnel's tap should be closed), add 40 mL of cold 10% (w/v) NaCl solution. Gently shake the mixture, and wait for the two phases to separate, discard the lower (aqueous) phase. Repeat the washing of the oil 3 more times, each with 40 mL of cold 10% NaCl solution, now with strong agitation (caution: periodically, release the pressure in the separation funnel). After each agitation, wait for 5-10 minutes for phase separation, and then slowly remove the (lower) aqueous phase. On the third wash, use a strip of pH paper to measure the pH of the aqueous phase. Repeat the washing if the pH is not equal to that of the NaCl solution, then wait for phase separation. Transfer the product to a 25 mL Falcon-tube, marked with the school’s name and group´s number, add two small spoons of anhydrous MgSO4 to the oil, agitate the tube gently and then give it to the TA.
|
2.2.6. Drying of the Biodiesel Samples and Calculation of Biodiesel Yield from Viscosity Measurement
- This part was carried out by the TAs after the student´s visit. Overnight, most of the samples in the Falcon tubes separate into an (upper) oil phases and a (lower) small aqueous phases. For each sample, the upper phase was transferred to a fresh, appropriately marked Falcon tube. These samples were then further dried under reduced pressure at 50°C (Isotemp model 281A, vacuum oven, Fisher brand) for 4 hours. The BD yields were calculated from the viscosity of the product (a mixture of BD + unreacted WCO) using a cone-plate rheometer (Brookfield model CAP2000+). The TAs prepared, by weight, mixtures of WCO and the corresponding BD-100%-WCO, ranging from (10% WCO + 90% BD-100%-WCO to 90% WCO + 10% BD-100%-WCO). The instructors measured the viscosity of these 9 samples, plus the starting WCO and the BD-100%-WCO; all measurements were carried out at 25°C. Plot of these viscosities versus wt% BD-100%-WCO in the mixtures showed a smooth curve (Figure 5, vide infra), from which the students calculated the BD yields of their experiments.
2.2.7. Other Activities During the Visits
- During the transesterification reaction, the laboratory technician demonstrated how gas chromatography equipment works, using Shimadzu GC-2014 FID equipment, the sample injected was a ethyl acetate solution of the oil of clove. Due to lack of time, the technician used this oil because of the relatively short retention time; < 8 minutes [17]. A previous gas chromatogram of BD-100% from coconut oil was also shown (retention time > 20 minutes).
3. Results and Discussion
3.1. Pre-Visit Questionnaire; Figure 1
- Below are some of the pre-visit questions given to the students and their answers:Q.1- What do you understand by sustainability?Q.2- Did you hear about green chemistry?Q.3- Did you hear about biofuels? Cite some examples. Q.4- What is the environmental impact of using biofuels? Cite two examples.
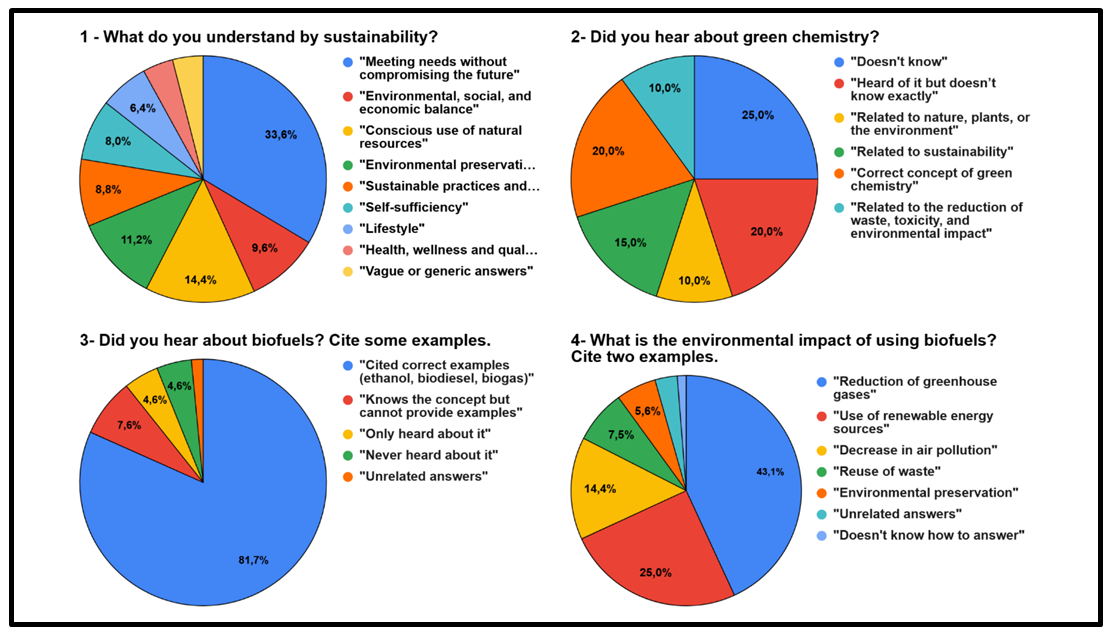 | Figure 1. Overview of the students’ answers to the pre-visit questionnaire |
3.2. Visits to the Institute of Chemistry of the University of São Paulo: Hands-on Experience in Recycling
- At the start of each visit, the students were shown Figure 2 for the starting materials and the corresponding (100%) BD samples. They were asked to subsequently discuss (in their schools) a possible (chemical) reason for the dark color of WCO, relative to that of fresh RPO. After explaining the scheme of activities, Figure 3, they were instructed on safety in the laboratory. We also explained that the transesterification reaction is an equilibrium, whose position (i.e., the BD yield) depends on the experimental conditions. The variables are the molar ratio EtOH/WCO, the reaction time and the catalyst concentration. The temperature is held constant (boiling point of EtOH). Then they went to the laboratory, see Figure 4.
 | Figure 2. The fatty material shown to the students at the start of their visit to Chem-USP. Here A, B, C and D refer to refined palm oil, waste cooking oil, 100%-biodiesel samples that we synthesized |
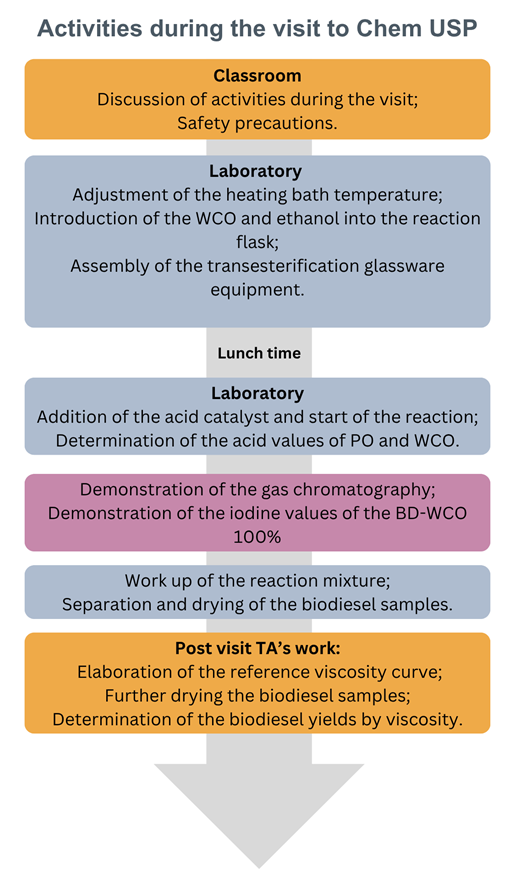 | Figure 3. Scheme of the activities of the green chemistry project |
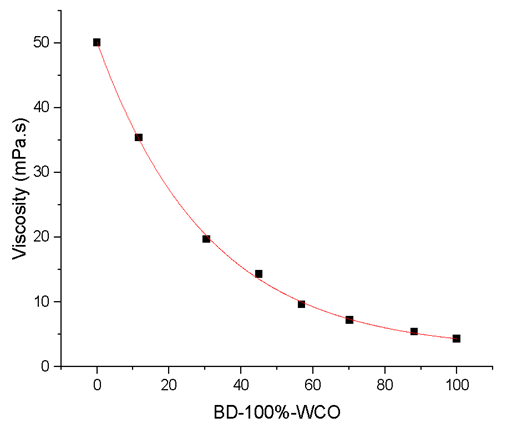 | Figure 5. Dependence of the viscosity of mixtures of (WCO + BD100%-WCO) on the weight percentage of (authentic) BD100%-WCO |
3.3. Post Visit Work
- The students discussed their results in the schools (PowerPoint). During these presentations, they were told that the dark color of the WCO is due to side reactions, e.g., oxidation, as shown, e.g., by Scheme 1. Note that RPO contains ca. 18% unsaturated fatty acids (oleic, linoleic and arachidic acids), hence is susceptible to oxidation at the high temperatures of frying [20] [21].
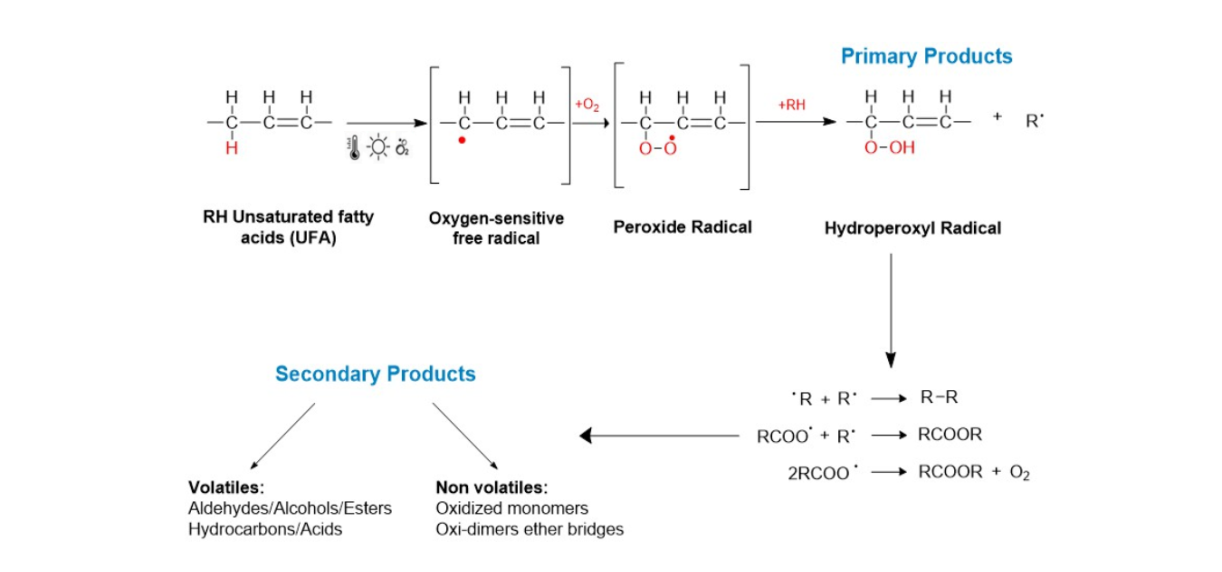 | Scheme 1. Typical side reactions that occur during repeated frying of food |
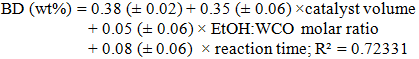 | (5) |
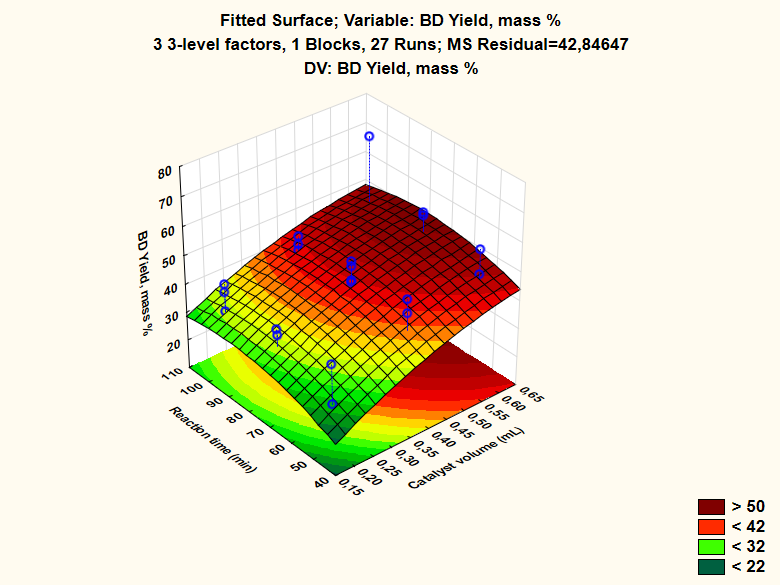 | Figure 6. Response surface for the dependence of BD yield (Z-axis) on catalyst concentration (X-axis) and reaction time (Y-axis) |
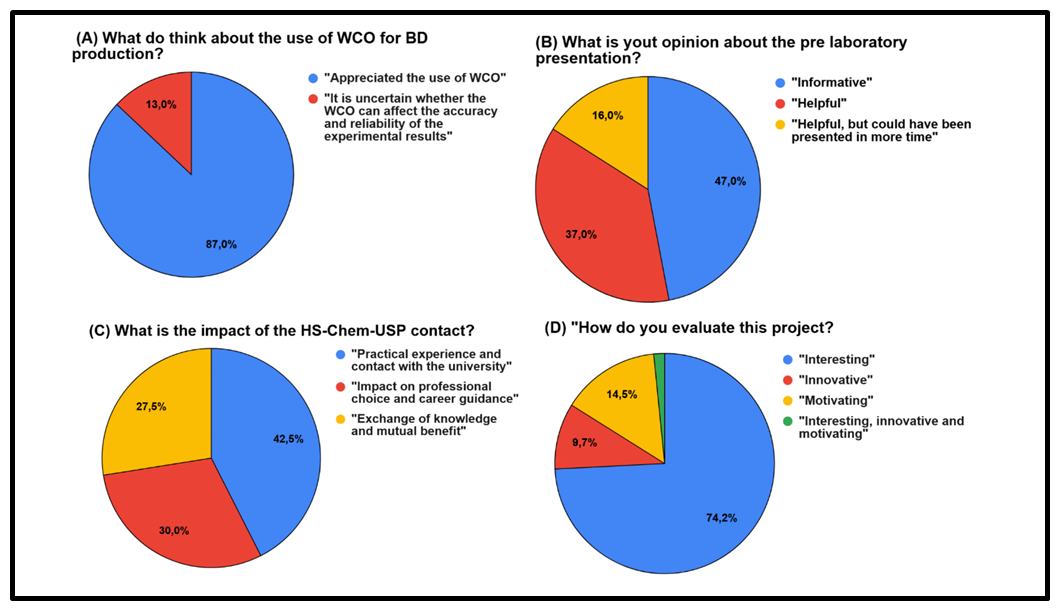 | Figure 7. Project´s evaluation by the students |
4. Conclusions
- The synthesis of biodiesel from WCO represents an interesting and simple “hands-on” approach to introduce HS students to recycling and green chemistry. An additional plus is the introduction of the students to food chemistry, including the properties of fresh oils, the changes caused by frying and to the reduction of the environmental impact of fuel burning. This project fits nicely into the education goal of the upcoming COP30 congress, as it increases the awareness of HS students about recycling as a strategy to synthesize BD. The use of this fuel results in less air pollution, an important aspect of green chemistry.
ACKNOWLEDGEMENTS
- We are grateful to the students who participated in this project. We thank Professor Dr. Pedro V. de Oliveira; Professor Dr. Carlos T. Hotta, and members of CCEx of Chem-USP for their support and encouragement; Mr. Alexandre S. Guarezemini (Chem-USP) for his competent technical support during all visits, and for demonstrating the gas chromatographic analysis, Mr. Cezar Guizzo for taking photos and Luciano Moreira and Andrea Pimentel, science coordinators, Luiz G. Righini State High School. We thank the administrations of the schools for their support of this activity. We acknowledge FAPESP (grants 2014/22136-4; 2025/04998-3) and CNPq (grant 306108/2019-4) for a research fellowship to Omar A. El Seoud and Nathália Peres, respectively.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML