-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2025; 13(1): 16-24
doi:10.5923/j.jlce.20251301.02
Received: Mar. 19, 2025; Accepted: Apr. 20, 2025; Published: Apr. 23, 2025

Synthesis and Composition of Tutton Double Salts: An Introductory Laboratory Project
Corissa P. McDonald, Claude H. Yoder
Department of Chemistry, Franklin and Marshall College, Lancaster, Pennsylvania, United States of America
Correspondence to: Claude H. Yoder, Department of Chemistry, Franklin and Marshall College, Lancaster, Pennsylvania, United States of America.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The synthesis and characterization of Tutton double salts is described as an ideal introduction to the synthesis and gravimetric analysis of soluble ionic compounds. The Tutton salts, with a formula of K2M(SO4)2•6H2O, where M is Mg, Zn, Cu, Ni, Co, and Fe, are prepared by mixing the simple salt “constituents” and then allowing for slow evaporation of the mother liquor. After a period of one day to one week, large (0.3-0.6 cm) monoclinic plates form and can be removed either by gravity filtration or by removal with tweezers. The stoichiometry of the compound is determined by gravimetric analysis of sulfate, the divalent cation, and water. The Tutton salts are of interest because of the variation in their color depending on the divalent cation, their crystal form and size, and the formation of a hydrate with water octahedrally coordinated to the divalent cation. The compounds lend themselves to discussions of the origin of colors in the transition metals, and coordination of water to the divalent cations. The Tutton salts also have enjoyed a variety of recent applications, such as their possible utility in heat exchange and UV filters, that may merit discussion as the instructor sees fit. The simplicity of the synthesis and the ease of isolation and characterization of the product make this an excellent introduction to the synthetic process and is suitable for both secondary and college courses. The synthesis and characterization of two Tutton salts containing different divalent cations can easily be performed by groups of two students in four two-hour lab periods. The techniques that each group is exposed to include precipitation, filtration, weighing, use of the Bunsen Burner for dehydration of the salt, and conversion of a hydroxide to the oxide by ignition in a crucible.
Keywords: Secondary chemistry laboratory, Introductory General chemistry, Individual or group experimental lab project, Double salts, Synthesis and characterization of double salts, Gravimetric analyses, Determination of water of hydration by heating
Cite this paper: Corissa P. McDonald, Claude H. Yoder, Synthesis and Composition of Tutton Double Salts: An Introductory Laboratory Project, Journal of Laboratory Chemical Education, Vol. 13 No. 1, 2025, pp. 16-24. doi: 10.5923/j.jlce.20251301.02.
Article Outline
1. Introduction
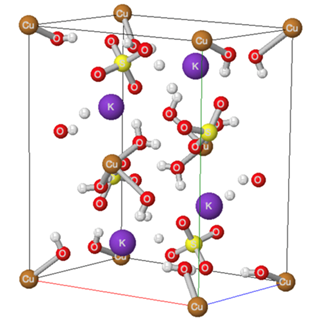
- Many inorganic materials can be categorized as double salts; that is, as an ionic compound that contains two or more different types of cations or two or more different types of anions. The common ore of copper, malachite, contains one type of cation and two different anions--hydroxide and carbonate (Cu2CO3(OH)2). Alums, with the general formula MAl(SO4)2 •12 H2O contain two different cations--generally an alkali metal cation (M) and an aluminum ion-- and only the sulfate anion. Many of the minerals in the earth's crust are double salts.In the early 1900s, the British crystallographer Alfred Edward Howard Tutton [1] studied a series of double salts that contains two different cations, generally an alkali metal cation (A+), a doubly charged cation (M2+), and the sulfate or selenate anion [2]. The monovalent cations (A+) are generally the relatively large alkali metal cations (potassium, rubidium, cesium) and the ammonium ion, while the divalent cations are usually the first-row transition metal +2 ions or Mg2+. Although the lighter alkali metal cations are present in double salts such as krohnkite (Na2Cu(SO4)2 • 2H2O) and bloedite (Na2Mg(SO4)2 • 4H2O) they appear not to form salts with the Tutton salt stoichiometry [3]. The general empirical formula for the Tutton salts, A2M(SO4)2•6H2O, where A is the monovalent cation and M is the divalent cation, can also be written as A2M(H2O)6(SO4)2, which emphasizes the association of the water molecules with the divalent cation.Several of the Tutton salts occur at geological features such as fumaroles, geysers, evaporite deposits, and ore tailings [4]. Their presence on Europa has been proposed [5] and picromerite, K2Mg(H2O)6(SO4)2, has been considered as a model of the primordial hydrosphere on Mars [6]. The mineral cyanochroite, K2Cu(H2O)6(SO4)2, has been the subject of several recent studies of the Jahn-Teller effect [7,8]. The easy synthesis, stability, and reversible endothermic removal of water of hydration of Tutton salts also makes them candidates for waste heat removal and recovery [9,10].One of the most interesting structural features of these salts, prepared in aqueous solution, is the coordination of water molecules to the divalent cation. The structure of cyanochroite (Figure 1), shows a monoclinic crystal with four potassium ions and four sulfate ions in the unit cell, which can be written as K4Cu2(H2O)12(SO4)4; that is, as twice the empirical formula. (Because the copper ions are shared with a number of other unit cells, the partial occupancy of the copper ion must be considered when determining the number in the unit cell). The number of water molecules coordinated with each copper ion is not obvious from Figure 1. This is more clearly indicated by Figure 2, where the octahedral arrangement of the six water molecules about each copper ion is shown as an octahedron.
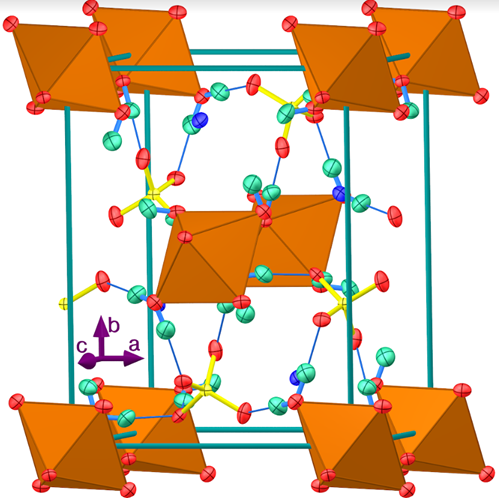 | Figure 2. The structure of cyanochroite, K2Cu(H2O)6(SO4)2, using copper-colored octahedra to portray the coordination of six water molecules around each copper ion [7] |
2. The Project
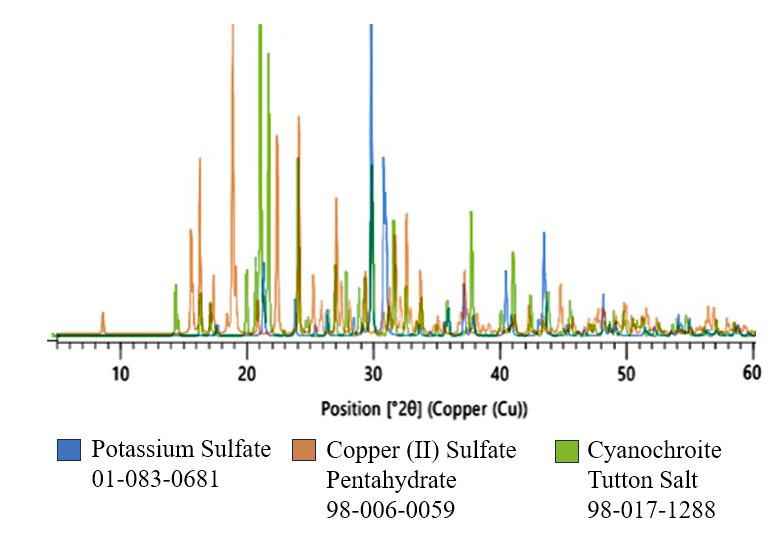
- We have found that the synthesis and analysis of Tutton salts make an excellent multi-period laboratory project suitable for both secondary and college chemistry courses. When the divalent cation is a transition metal cation, the salts, except for those of Zn2+, are colored. These salts provide a way to describe and discuss a number of interesting concepts and techniques. For example, the colors of the salts can be discussed at a variety of levels, from the simple observation that many transition metal compounds are colored to a rationalization of color using electron configuration and energy levels. The coordination of water molecules to the divalent cation is particularly interesting and invites a discussion of electron-sharing (Lewis acid-base) reactions and the models used to describe those interactions. Of course, the level at which this coordination is discussed will depend on the course and the instructor but could certainly involve the valence bond model with hybridization of orbitals, or, in some courses, the use of the crystal field model might be desirable, particularly as an alternative model.After the compound is synthesized, the composition; that is, the identity of the ions and their stoichiometry in the solid, crystalline compound must be established before the bonding within the compound and its physical properties can be adequately described. Techniques such as X-ray diffraction (XRD) and infrared spectroscopy (IR) are frequently used to determine the identity of groups within the structure and to establish that the compound obtained in the synthesis is not a mixture of starting materials and has been previously described in the chemical literature. The XRD line pattern of the potassium/copper (cyanochroite) Tutton salt and XRD patterns of the simple salts used to prepare the Tutton salt are shown in Figure 3. Although cyanochroite, K2Cu(H2O)6(SO4)2, could be imagined as a double salt consisting of K2SO4 and CuSO4 (and 6 H2O), the XRD pattern of the Tutton salt is different from that of these simple salt “constituents.” The internal structure of the double salt therefore is not simply a mixture of simple salts.
2.1. Synthesis
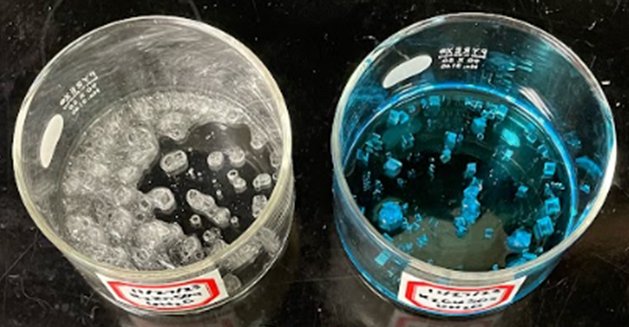
- The Tutton double salts are prepared by simply mixing saturated solutions of the simple salts and allowing slow evaporation of the water in the mixture to produce large (ca. 0.2-0.5 cm) monoclinic crystals. The crystals of K2Cu(H2O)6(SO4)2 and K2Co(H2O)6(SO4)2 are shown in Figure 4. The dry crystals are stable and can be easily dissolved for analysis. Purification of the prepared salts is difficult, however, due to the high solubility of the double salts. The immediate removal of the crystals upon formation from the simple salt mixture serves as the most consistent regulation of purity. In our experience, purification is not necessary for the accuracy of the compositional analyses.
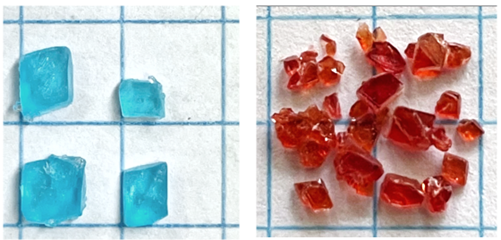 | Figure 4. Crystals of K2Cu(H2O)6(SO4)2 (left) and K2Co(H2O)6(SO4)2 (right) obtained by slow evaporation of mixtures of the simple salts. Background grid line separations are 1 cm |
2.2. Composition
- The composition of the salts can be determined using quantitative analyses followed by a classroom discussion of the difference between an empirical formula and a structural formula. For example, the analyses should allow a student to determine that the empirical formula of cyanochroite is approximately K2Cu(H2O)6(SO4)2, but these techniques cannot establish the coordination of the six water molecules to the copper cation.The characterization procedures for these compounds are illustrated below for cyanochroite:(1) Determination of the percentage of the divalent cation by precipitation of the divalent hydroxide followed by conversion of the hydroxide to the oxide by heating (2):
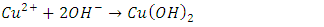 | (1) |
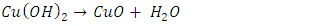 | (2) |
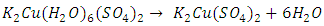 | (3) |
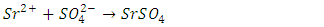 | (4) |
2.3. Laboratory Time Requirements
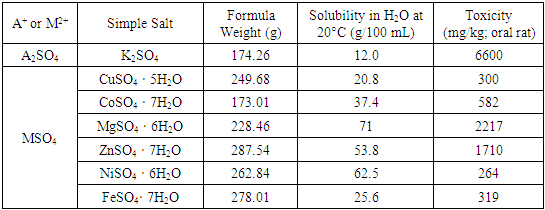
- At least three laboratory periods are required for this project. The Tutton salt is synthesized in the first laboratory period. The time required for the synthesis is controlled by the amount of time required to make a saturated solution of the individual simple salts. Table 1 provides a guide to the solubility of the simple salts and with gentle warming the time required can be as low as 30-45 minutes to prepare and mix the simple salts. The formation of the double salt requires several hours to one week of slow evaporation, depending on the nature of the cation. Isolation of the double salt with drying on filter paper requires no more than 30 minutes. Depending on the nature of the laboratory course, students may prepare double salts in one laboratory period and isolate the crystals from the mother liquor a week later. The total time required for characterization, including both gravimetric determinations of sulfate and the divalent cation, is approximately four hours. Because the strontium sulfate precipitate requires a week to dry and the copper oxide must be cooled to room temperature, a third laboratory period may be utilized to perform tests such as determination of water (one hour), the flame test for potassium and divalent cation (15 minutes), the ammonia complex test for copper (II) (15 minutes), and calculation of the empirical formula.
3. Methods and Materials
3.1. Syntheses
- All simple salt solutions were prepared using Milli-Q deionized water and ACS Reagent grade reagents with purities above 97%. The simple salts were mixed in a 1 to 1 mole ratio, which in our experience minimizes contamination of the product. The amounts of these reactants were determined from their solubilities in water at 20°C (Table 1).
|
4. Characterization
4.1. Color
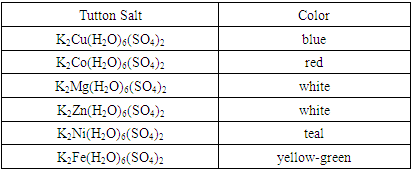
- Crystals were ground into a powder before analysis. The colors of the potassium salts are shown in Table 2.
|
4.2. X-Ray Diffraction
- Double salts were characterized by X-ray diffraction using a PANalytical X'pert PRO Multipurpose diffractometer Theta-Theta System with CuΚα radiation (λ = 1.54060 Å). The results were analyzed using the PANalytical program X'Pert Highscore Plus. The diffraction pattern of cyanochroite is shown in Figure 6, along with the best literature match. Simple sulfate salt diffraction patterns were shown previously in Figure 3.
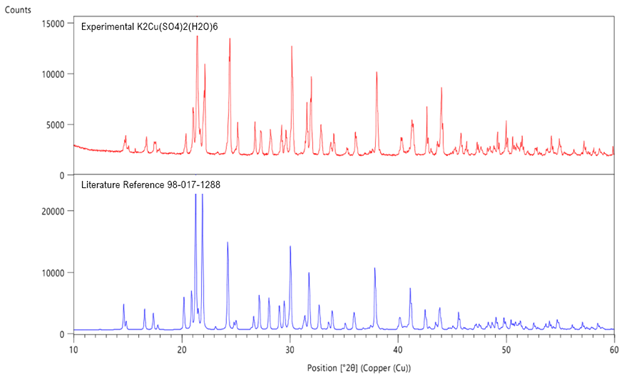 | Figure 6. XRD pattern for the prepared, K2Cu(H2O)6(SO4)2 (top) and comparative literature [15] pattern (bottom) |
4.3. Qualitative Identification of Ions Present in the Tutton Salt
- The ions present in the Tutton salt can be identified using a few simple procedures. The presence of potassium and copper can be identified with a flame test, while the presence of sulfate can be determined by precipitating SrSO4 using a 0.1 M Sr(NO3)2 solution from an aqueous solution of the Tutton salt. The presence of water in the dry salt can be ascertained by heating the salt in a test tube over a Bunsen flame. In each test, the results of a control test should be compared to the target analysis. This is particularly important in the flame test where impurities such as the ubiquitous sodium ion produce an orange flame, which obscures the flame of the target ion or produces a misleading color.
4.4. Quantitative Analysis of Water of Hydration
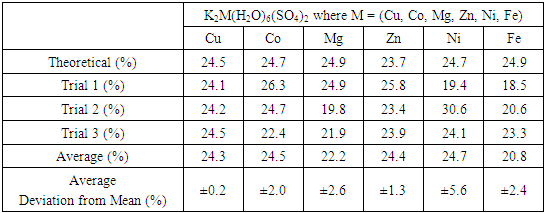
- A small sample (0.1-0.25g) of finely powdered crystal was weighed into a 100 mm test tube and the test tube (and contents) were reweighed. The test-tube, held in a test-tube holder, was heated in a relatively hot (inner blue cone present) Bunsen flame to drive off the water present in the crystal. To remove all the condensate, the test tube was held at about a 45° angle and moved slowly through the flame for 10-15 seconds. During this time, the test tube was moved up and down to heat all parts of the tube. This heating process was repeated several times to ensure that the water was removed from both the powder and the tube. After the test tube cooled slightly, it was capped with a cork and cooled to room temperature and reweighed without the cork. The percentage of liberated water was then compared to the theoretical percentage of water derived from the empirical formula for each salt. Duplicate measurements on cyanochroite gave an average of 1 percent error relative to the theoretical. The average percentage error for all the Tutton salts as reported in Table 3 is 5.3.
|
4.5. Qualitative Analysis of Sulfate
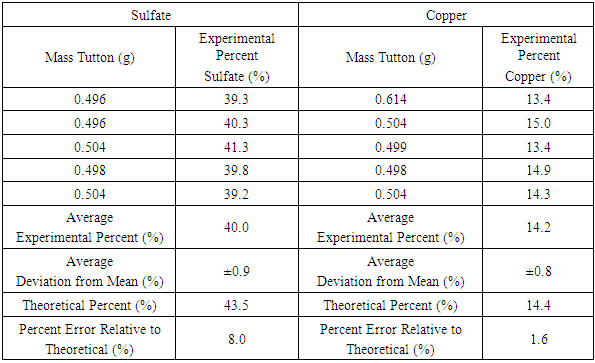
- The standard gravimetric procedure for the analysis of sulfate involves the precipitation of the low solubility BaSO4. Because SrSO4 is only slightly less insoluble (Ksp = 10-7 compared to 10-10 for BaSO4), and Sr(NO3)2 is less toxic (LD50 1892 mg/kg oral rat) than Ba(NO3)2 (LD50 355 mg/kg oral rat) the precipitation of Sr(NO3)2 was used. A sample (0.450-0.650 g) of finely powdered double salt was added to 50 mL of distilled water in a 100 mL beaker. A hundredth mole sample of Sr(NO3)2 was dissolved in 100 mL of distilled water to prepare a 0.1 M solution. Using a 50 mL burette, approximately 25 mL of the strontium nitrate solution was added dropwise at a rate of 2 drops/s to the double salt solution while heating to about 60°C and stirring magnetically. The mixture was allowed to sit with occasional stirring with a glass stir rod for about 30 minutes before being covered with a watch glass and left undisturbed for 15 minutes or for up to one week. The mixture was then vacuum filtered through a Buchner funnel containing a previously weighed 5 cm quantitative filter paper. After drying the precipitate (on the filter paper) in a 100°C oven, the precipitate (and paper) was weighed again to obtain the mass of precipitated SrSO4 (Table 4). The filtration can also be done by gravity. It is imperative to preserve the filtrate for further characterization analyses.
4.6. Quantitative Analysis of Copper
- The percentage of the divalent cation can be determined by a variety of techniques, such as conversion to the oxide and colorimetry, but a simpler method makes use of the insolubility of the hydroxide. Because of the high solubility of the double salt, a hydroxide solution can be added directly to a weighed amount of the double salt until the hydroxide is completely precipitated as indicated by a change in pH from that of the slightly acidic (ca pH = 5) double salt solution to the basic solution produced after total precipitation of metal hydroxide. In the case of some cations, the product may also be a double salt containing the anion present during the precipitation. This is the case with copper (II) sulfate double salts, for which the addition of hydroxide, under some conditions, produces brochantite, Cu4SO4(OH)6 [12,13]. To alleviate this problem, 0.10 M NaOH hydroxide is added after the removal of the sulfate ion by precipitation of SrSO4.Following the removal of sulfate, the filtrate (see above) is stirred magnetically in a 250 mL beaker. 25-30 mL of 0.1 M hydroxide solution is added to the filtrate with a burette using a drop rate of about 2 drop/s. After 15 minutes, the mixture is vacuumed filtered through a Buchner funnel using a previously weighed 5 cm quantitative ashless filter paper. The precipitate and filter paper are then placed in a porcelain crucible and heated strongly over a Bunsen flame to convert the precipitated hydroxide to the more stable oxide, which is then weighed. Ashless filter paper is strongly recommended for the most accurate quantitative determinations. The copper and sulfate results are compared with the theoretical percentages based on the empirical formula for cyanochroite in Table 4. Uncertainties reported are the average deviations from the mean of duplicates, given as a percent of the mean. Separate portions of the same sample must be used for all three quantitative tests.
|
4.7. Calculation of Formula
- The composition of the cyanochroite Tutton salt can be determined from the three quantitative analyses (Cu, SO4 and H2O) by assuming that the remainder of the double salt is potassium The number of moles of K+, Cu2+, SO42- and H2O in 100 g of cyanochroite as determined from the experimental and theoretical percentages is shown in Table 5.
5. Discussion
- The objective of the project is to synthesize and determine the composition of the synthesized Tutton salt. This composition is expressed by the empirical formula of the compound, which provides the molar relationship between the elements and groups that constitute the compound. The Tutton salts are especially interesting because of the location of the water molecules in the compound. That location is not expressed by the empirical formula, but rather by the structural formula, which indicates the connections between the elements or groups. Such information must be derived from other techniques such as infrared or nuclear magnetic resonance spectroscopy. The relationship between the empirical formula and the structural formula can be extracted by the question—where are the water molecules in the solid compound? This question should evoke discussions of the interactions in an ionic compound: electrostatic forces expressed by the Columb relationship. In a hydrate, however, there are additional interactions brought about by the dipoles and hydrogen bonding of the water molecules, as well as the very real possibility of covalent interactions between the water molecules and the ions. Thus, in an ionic hydrate such as the Tutton salts, the student should envision the forces that are present in the solid as ion-ion interactions, dipole-dipole interactions among the water molecules, dipole-ion interactions between the ions and water molecules, possible covalent interactions between the ions and water molecules, as well as the ubiquitous van der Waals forces. After this variety of forces is expressed, the question of how the water molecules might interact with the ions can be addressed (see Figure 7).
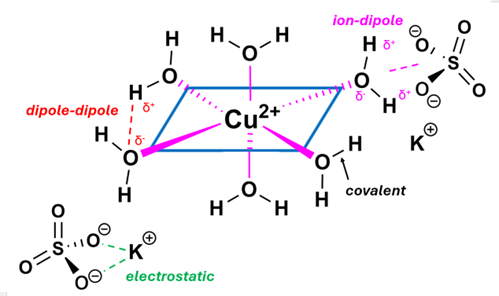 | Figure 7. Typical electrostatic and covalent interactions in a Tutton Salt. Hydrogen-bonding between hydrogen and lone pairs of electrons on the oxygens of neighboring species, and Van der Waals (dispersion) forces are not shown |
6. Supplementary Material: For the Instructor and Student
- All samples should be prepared using distilled or deionized water and ACS Reagent grade reagents with purities above 97%. For the synthesis of the Tutton salt, students should have access to two 30-50 mL beakers, a crystallizing dish, a 60 mm wide polystyrene or glass funnel or Buchner funnel, 50- and 110-mm filter paper, 125-250 mL Erlenmeyer flask, 10 mL graduated cylinder, and a glass stirring rod. A hot plate will facilitate the dissolution of the simple sulfates. The materials needed for characterization tests are listed in subsequent sections.
6.1. Synthesis of a Tutton Salt
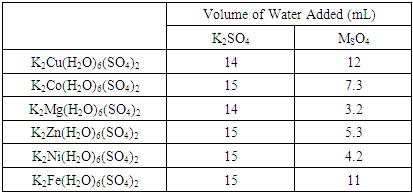
- Instructors may find it helpful to have students use the solubility data given in Table 6 to determine the amounts of reagents to use to prepare saturated solutions. The data in the ‘Mass reagents” and “Volume” columns of Table 6 are the masses of the A2SO4 and MSO4 reagents and the volume of water that we recommend for the preparation of the saturated solutions. The column “mole reactant” shows that the 1:1 mole ratio between the monovalent and divalent sulfate reactants must be maintained.
|
6.2. Characterization: Qualitative Tests
6.2.1. Flame Test and Sulfate Test
- The air collar of a Bunsen burner should be opened to provide an inner blue cone of flame. A six-inch Nichrome wire can be used for the flame test but must either be inserted in a glass tube by melting the tube around the end of the wire or attached to a spatula by winding about 2 inches around the end of the spatula. It may be desirable to make a small loop at the end of the wire to allow the wire to retain powders more easily. The last one inch of the Nichrome wire must be cleaned by heating it at the tip of the inner blue cone of the Bunsen flame. This will require a minute or two of heating if badly contaminated. The cleaning process can be hastened by dipping the wire into a few drops of 3 M HCl and then reheating. A small sample (as a powder) of the Tutton salt should be placed in a beaker or spot plate. The Nichrome wire is then dipped into the Tutton salt powder and then placed into the top portion of the Bunsen flame. If potassium is present, a pale violet color will develop in the flame after a few seconds. If copper is present, a blue-green flame will develop. Other divalent ions in Tutton salts do not produce readily visible colors in the flame. A bit of sparkling does not indicate the presence of a particular ion. It is always good practice to run a control test. For example, if copper is suspected, the flame test should be run on a sample of CuSO4 or some other known copper salt. Because the flame may be contaminated by other ions, a control should also be run with distilled water, which should impart no color to the flame. Note: A drop of 3 M HCl will enhance the flame because of the greater volatility of the chlorides of the dipositive metal ions.The same sample of the Tutton salt can also be used to determine if the sulfate ion is present. Sulfate will react with either Ba2+ or Sr2+ to form insoluble BaSO4 (5) or SrSO4 (6).
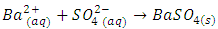 | (5) |
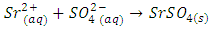 | (6) |
6.2.2. Ammonia Complex Test
- If the salt contains a divalent cation that forms an ammonia complex, the addition of 3 M ammonia (NH3 gas dissolved in water) will initially form the solid hydroxide and then, upon addition of more ammonia, will dissolve the hydroxide and form an ammonia complex. This Lewis acid-base reaction occurs with Cu2+ even when the ammonia solution is fairly dilute (like 3 M NH3), and with Co2+ when 6 M NH3 is used. With both ions, the first stage of the reaction is the formation of hydroxide, followed by dissolution of hydroxide to form the complex ion. These Lewis complexes are soluble in water and strongly colored: (Cu(NH3)42+ is dark violet-blue and Co(NH3)62+ is blue. The Co2+ ion oxidizes easily in air. For the copper cation, the reactions are shown below (7,8)
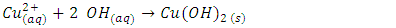 | (7) |
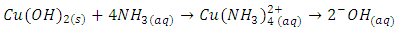 | (8) |
6.2.3. Waters of Hydration
- Place a small spatula-full of Tutton salt in a 100 mm test tube and heat gently in a Bunsen flame. The gas jet should be regulated to produce a flame that extends about 3 to 4 inches. The air-inlet collar of the Bunsen burner should be opened enough to create an inner blue cone. Using a test tube holder to hold the test tube, move the tube into the edges of the flame to see if any water gathers at the crystals. Almost all hydrates will decompose with heating into the anhydrous salt and water.
6.3. Characterization: Quantitative Procedures
6.3.1. Quantitative Analysis of Sulfate: Precipitation of SrSO4
- A 0.45-0.65 g sample of finely powdered double salt is used for both sulfate and copper quantitative analyses. This sample is dissolved in 50 mL of distilled water in a 100 mL beaker. The amount of the sulfate ion in the sample can be obtained by the precipitation of strontium sulfate as shown below. Instructors may prepare a 0.100 M Sr(NO3)2 solution in advance or ask students to prepare a 50 mL solution themselves. Students should fill a 50 mL burette with approximately 30-35 mL of the tenth molar solution, which is added dropwise to the Tutton salt mixture. Following the transfer of the precipitate, it is necessary to preserve the filtrate for further analyses. If the filtrate is discarded, students cannot complete the gravimetric determination of copper.
6.3.2. Quantitative Analysis Divalent Cation
- The amount of the divalent cation in the sample can be obtained by the slow addition of hydroxide to the filtrate obtained in the determination of sulfate (above). Instructors should prepare the 0.10 M NaOH solution in advance and convey the hazards involved in working with a strong base to the students. The amount of hydroxide added is determined by the 1:2 stoichiometric ratio between Cu2+ and OH- in Cu(OH)2. In the final step, the Cu(OH)2 precipitate is ignited in a crucible using a Bunsen flame to convert the copper hydroxide to the more stable copper oxide. The reaction is shown below.
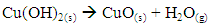 | (9) |
6.3.3. Water of Hydration
- A sample of 0.1-0.25 g of the finely powdered crystal was weighed (to three decimal places) into a 100 mm test tube. The test tube and contents must then be reweighed. A Bunsen burner flame should be adjusted so that it is no more than 4 inches high with an inner darker blue cone. The inner cone is controlled by the amount of air admitted through the Bunsen collar. Students should be trained in the use of an open flame with appropriate cautions about loose hair and other flammables. The test tube containing the crystal sample should then be placed at the outer edges of the flame to heat the sample gently. When moisture is seen inside the tube, the tube should be moved closer to the middle of the flame for several seconds and then the entire tube must be heated from bottom to top so that all of the water that condenses in the tube is driven off. The whole heating process should not take longer than about 2 minutes. The process of removing the condensate should then be repeated at least one more time. After the tube has cooled somewhat it should be capped with a cork to prevent moisture from entering. After the test tube and contents have returned to room temperature the test tube (and contents) is reweighed (without the cork). To ensure all water has been removed, the test tube may be heated again, cooled to room temperature, and reweighed. Students may repeat this process until a constant weight is obtained; however, the procedure previously outlined (See Materials and Methods 4.4) requires only one heating for driving off all waters of hydration. Instructors may opt to use a crucible for larger amounts of sample, although the small test tube is optimal for safe heating of less than 150 mg. The mass of the anhydrous salt, the mass of the evolved water, and the percentage of water in the double salt can then be determined. This percentage should be compared to the theoretical percentage of water in the Tutton salt according to its formula. If a drop of water is added to the anhydrous product the product will return to its initial state.
6.3.4. Calculation of Formula
- The composition of the salt can be determined from the three quantitative analyses (copper, water and sulfate-) by assuming that the remainder of the double salt is potassium. If the laboratory results give 40% SO4, 14% Cu and 25% H2O, these can be converted into moles per hundred grams:
 This can be used to obtain the ratio of moles of each species relative to copper (II).
This can be used to obtain the ratio of moles of each species relative to copper (II).  Rounding the ratio of moles within the uncertainty of the work:
Rounding the ratio of moles within the uncertainty of the work:  Thus, the formula is K2Cu(H2O)6(SO4)2 which is consistent with the empirical formula of cyanochroite.
Thus, the formula is K2Cu(H2O)6(SO4)2 which is consistent with the empirical formula of cyanochroite. ACKNOWLEDGEMENTS
- The authors are indebted to Emily Wilson of Franklin and Marshall College for technical assistance, and to the donors of the Yoder Research Fund.
DISCLOSURE
- The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
| [1] | Partington, J. R. (1938). Dr. A. E. H. Tutton, F. R. S. Nature, 142(3590), 321–322. https://doi.org/10.1038/142321a0. |
| [2] | Tutton, A. E. (1896). Connection between the atomic weight of contained metals, and the crystallographical characters of isomorphous salts. The volume and optical relationships of the potassium, rubidium, and cæsium salts of the monoclinic series of double sulphates, R2M(SO4)2.6H2O. J. Chem. Soc., Trans., 69(0), 344–495. https://doi.org/10.1039/CT8966900344. |
| [3] | Weil, M. (2013). On the (non-)existence of Tutton salts with formula types [M(H₂O)₆](ClO₄)₂(H₂O)₂ and Na₂M(SO₄)₂(H₂O)₆ (M is a first-row transition metal). Acta Crystallographica Section C, 69(9), 990–994. https://doi.org/10.1107/S0108270113020027. |
| [4] | Bosi, F., Belardi, G., & Ballirano, P. (2009). Structural features in Tutton’s salts K₂[M2+(H₂O)₆](SO₄)₂, with M2+ = Mg, Fe, Co, Ni, Cu, and Zn. American Mineralogist, 94(1), 74–82. https://doi.org/10.2138/am.2009.2898. |
| [5] | Zoltov, M. Y., & Shock, I. I. (1990). A hydrothermal origin for the sulfate-rich ocean of Europa, Lunar and Planetary Science XXXII [Abstract]. https://ntrs.nasa.gov/search.jsp?R=20010045108. |
| [6] | King, P. L., Lescinsky, D. T., & Nesbitt, H. W. (2004). The composition and evolution of primordial solutions on Mars, with application to other planetary bodies. Geochimica et Cosmochimica Acta, 68(23), 4993–5008. https://doi.org/10.1016/j.gca.2004.05.036. |
| [7] | Peets, D. C., Avdeev, M., Rahn, M. C., Pabst, F., Granovsky, S., Stötzer, M., & Inosov, D. S. (2022). Crystal Growth, Structure, and Noninteracting Quantum Spins in Cyanochroite, K2Cu(SO4)2•6H2O. ACS Omega, 7(6), 5139–5145. https://doi.org/10.1021/acsomega.1c06143. |
| [8] | Aramburu, J. A., Bhowmik, A., Garcia-Lastra, J. M., Garcia-Fernandez, P., & Moreno, M. (2019). Insight into Compounds with Cu(H2O)62+ units: New ideas for understanding Cu2+ in Tutton Salts, J. Phys. Chem. C, 123(5), 3086-3101. https://doi.org/10.1021/acs.jpcc.8b10441. |
| [9] | Fouda, A. E., Despault, G. J. G., Taylor, J. B., & Capes, C. E. (1980). Solar storage systems using salt hydrate latent heat and direct contact heat exchange—I preliminary design considerations. Solar Energy, 25(5), 437–444. https://doi.org/10.1016/0038-092X(80)90451-X. |
| [10] | Morales, A., Cooper, N., Reisner, B. A., & DeVore, T. C. (2022). Enthalpies of formation and standard entropies for some potassium Tutton salts. Chemical Thermodynamics and Thermal Analysis, 8. https://doi.org/10.1016/j.ctta.2022.100085. |
| [11] | Crystal Structure of Cyanochroite. Adapted from “Structural features in Tutton's salts K2[M2+(H2O)6](SO4)2, with M2+ = Mg, Fe, Co, Ni, Cu, and Zn” by Bosi F, Belardi G, & Ballirano P (2009) American Mineralogist, 94, 74-82. . |
| [12] | Yoder, C. H., Agee, T. M., Ginion, K. E., Hofmann, A. E., Ewanichak, J. E., Schaeffer, J. C. D., Carroll, M. J., Schaeffer, R. W., & McCaffrey, P. F. (2007). The relative stabilities of the copper hydroxyl sulphates. Mineralogical Magazine, 71(5), 571–577. https://doi.org/10.1180/minmag.2007.071.5.571. |
| [13] | Brigandi, L. M., Leber, P. A., & Yoder, C. H. (2005). Synthesis and Analysis of Copper Hydroxy Double Salts. J. Chem. Educ., 82(11), 1662. https://doi.org/10.1021/ed082p1662. |
| [14] | Leocádio, R. R. V., Perpétuo, G. J., Franco, C. J. & Batista, A. C. (2023). Growth and structural characterization of Tutton salt mixed of Co and Ni. REM: International Engineering Journal, 76(1), 55-62. https://doi.org/10.1590/0370-44672020760009. |
| [15] | Simmons, C. J., Stratemeier, H., Hitchman, M. A., & Riley, M. J. (2006). Influence of lattice interactions on the Jahn-Teller distortion of the [Cu(H2O)6]2+ ion: dependence of the crystal structure of K2[Cu(H2O)6]SO4(2x)(SeO4)(2-2x) upon the sulfate/selenate ratio. Inorganic Chemistry, 45(3), 1021–1031. https://doi.org/10.1021/ic050790j. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML