José A. Bravo, Juan José Dorado, Rodrigo Villagómez, Alberto Calle, José L. Vila
Instituto de Investigaciones Químicas, Universidad Mayor de San Andrés, 1995 Villazón ave., La Paz, Bolivia
Correspondence to: José A. Bravo, Instituto de Investigaciones Químicas, Universidad Mayor de San Andrés, 1995 Villazón ave., La Paz, Bolivia.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
We present a lab guide of the Friedel-Crafts (F-C) acylation (synthesis of aromatic ketones) for its application in undergraduate teaching laboratories of organic chemistry as part of the educational series in organic synthesis: “The Organic Chemistry Notebook Series, a Didactical Approach”. This guide is enriched by a short bibliographic-historical on the F-C reaction including proposals on mechanistic views. The synthesis of acetophenone (1) from benzene and p-methylacetophenone (2) from toluene was carried out using aluminum chloride as Lewis acid and acetyl chloride and acetic anhydride as acylating agents, respectively. The introduction of new technologies/instrumentation into educational laboratory settingscould be seen inthe identification of the products that was carried out by gas chromatography coupled with mass spectrometry (GC-MS) not of common use in undergraduate laboratories nowadays, the sample quantity ranged between 1 to 3 mL. This technique is more easily accessible and less expensive than 1H/13C NMR analysis and has the additional advantage of quantifying the product (yield) and assessing its purity, sometimes allowing impurities to be characterized. The results obtained present the somewhat distinctive feature of high and medium yields and purity of the synthesized products (1 and 2).
Keywords:
Friedel-Crafts Acylation, Organic Chemistry Lab, Synthesis, Mechanistic Approach, Aromatic Ketones, GC-MS, Chemistry Didactics
Cite this paper: José A. Bravo, Juan José Dorado, Rodrigo Villagómez, Alberto Calle, José L. Vila, Friedel-Crafts Acylation, Lab Guide, Use of GC-MS, The Organic Chemistry Notebook Series, a Didactical Approach, N°17, Journal of Laboratory Chemical Education, Vol. 13 No. 1, 2025, pp. 1-15. doi: 10.5923/j.jlce.20251301.01.
1. Introduction
Charles Friedel (1832-1899), French chemist born in Strasbourg, and James Mason Crafts (American chemist, Boston, MA, 1839-1917) authored in 1877 three short communications of Comptes Rendues about the synthesis of aromatic ketones [1-3]. This reaction named Friedel-Crafts acylation reaction [4], is a powerful and industry employed reaction from then until the present. This contemplates the use of AlCl3, an inexpensive and recoverable catalyst.The account of the historical and technical evolution of Friedel-Crafts’ alkylation and acylation was detailed in the publication by Wisniak (2009) [4]. The way to the affination of the F-C reaction debuted with the heat treatment of some alkyl or acyl chlorides with metallic aluminum, a low-rate reaction. Then, the authors changed metallic Al for aluminum chloride, giving a fast reaction at low temperature with evolving gases (HCl and CnH2n+2). This panorama let CF and JC envisage a high potent method for the synthesis of hydrocarbons or their oxygenated derivatives (ketones). A first mechanism attempt was proposed by CF and JC, with AlCl3 in the catalyst role, i.e. used and recovered. Such hypothesis was based on the appearing of a small quantity of a phenyl aluminum compound (supposed to be C6H5•Al2Cl5). This intermediate reacted with the alkyl chloride to afford a new hydrocarbon secundum: C6H5•Al2Cl5 + RCl Ò C6H5R + Al2Cl6, [4]. This reaction can be broken down by means of the mentioned mechanism as shown in Figure 1. 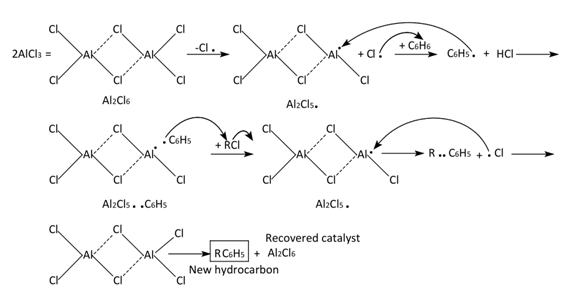 | Figure 1. Benzene/Alkyl Chloride Coupling Reaction, Mechanism |
The reaction takes place by interaction of the catalyst in its dimeric form. The excision of a Cl atom from the aluminum chloride provokes extraction of an H atom from benzene to afford HCl. The coupling between C6H5 and Al2Cl5 occurs and the adduct is formed. The Al-catalyzed species is now well-fitted for an attack over the organic chloride to generate a new C-C bond, or said otherwise a new hydrocarbon RC6H5 and subsequent recovery of the aluminum catalyst. In a posterior communication [4,5], FC and JC proposed a correction of their mechanism however it implied the consumption of AlCl3 during reaction [4].Later on, CF and JC investigated other possibilities of aluminum halides as catalysts. For example, the interchange of chloride by iodide in amyl chloride as shown in Figure 2 [4,6]. 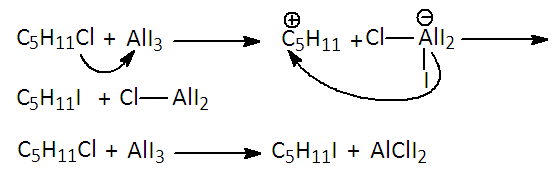 | Figure 2. Use of Previously Prepared AlI3 during Iodide Chloride Interchange in Hydrocarbons (Mechanism and Global reaction) |
From these experiments CF and JC envisaged the possibility of given the presence of a benzene ring the interaction between this with the halogenated hydrocarbon would afford a mixture of both greasy moieties as the result of the coupling between them in a new hydrocarbon weighing the sum of the parts. Such result was possible thanks to the presence of aluminum chloride [4,5], see Figure 3.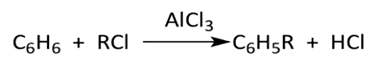 | Figure 3. Friedel-Crafts Alkylation, Catalyzed by AlCl3 |
Friedel-Crafts reaction originally intended for the synthesis of halogenated aliphatic compounds with benzene with the catalytic competition of aluminum trichloride, (cf. Figure 4 [7]), was similarly employed to synthesize aromatic ketones produced from the coupling of an aliphatic or aromatic acid chloride (instead of an alkyl chloride) with an aromatic hydrocarbon (e.g. benzene), Figure 4. 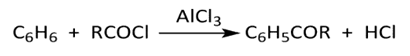 | Figure 4. Friedel-Crafts Acylation |
Other assays on acylation by CF and JC [4,5] included the attachment of other species (e.g. acid anhydrides) to benzene or its homologues according to the generic reaction C6H6 + O + Al2Cl6 = C6H5O + Al2Cl5 + HCl. The supposed mechanism involved evolution of HCl from AlCl3 (experimentally proven) [4]. Figure 5.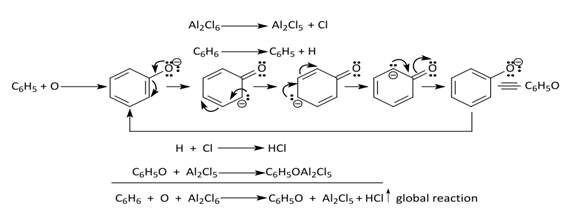 | Figure 5. Other Assays by Friedel and Crafts |
The mixture of benzene or toluene and aluminum chloride was exposed to airby oxygen giving rise to phenol (C6H5OH) or cresol (HOC6H4CH3), respectively after addition of water. Also, the procedure employing S, CO, SO2, or CH2=CH2 instead of O produced C6H5SH (thiophenol), C6H5COOH, C6H5SO2H (phenyl sulphinic acid), C6H5CH2CH3, C6H4(CH2CH3)2 or C6H3(CH2CH3)3. Conclusively, CF and JC proposed the resumed mechanism as shown in Figure 6 [4].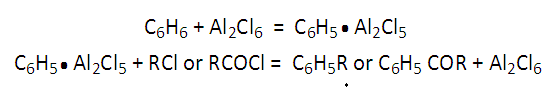 | Figure 6. Alkylation or Acylation Mechanism as Proposed by CF and JC [4] |
We present a lab guide of the Friedel-Crafts (F-C) acylation (synthesis of aromatic ketones) for its application in undergraduate teaching laboratories of organic chemistry as part of the educational series in organic synthesis: “The Organic Chemistry Notebook Series, a Didactical Approach”. The lab guide conducts to the synthesis of acetophenone (1) as well as 4-methylacetophenone (2). Mechanistic views are provided for some reactions. Also, innovation is intended for laboratory practices by means of the application of GC-MS technique for identifying the obtained ketones and minor derivatives; this is suitable given that both products are liquid at room temperature.
2. Friedel-Crafts Acylation Lab Guide
This lab guide comprises two parts, materials and reagents section, and experimental procedure section. These items must be available and correctly assembled in conformity to the Experimental Procedure Section below before the practice should be get started.
2.1. Reagents and Materials Section
2.1.1. Reagents’ Data, Table 1
Table 1. F-C Reaction, Reagents
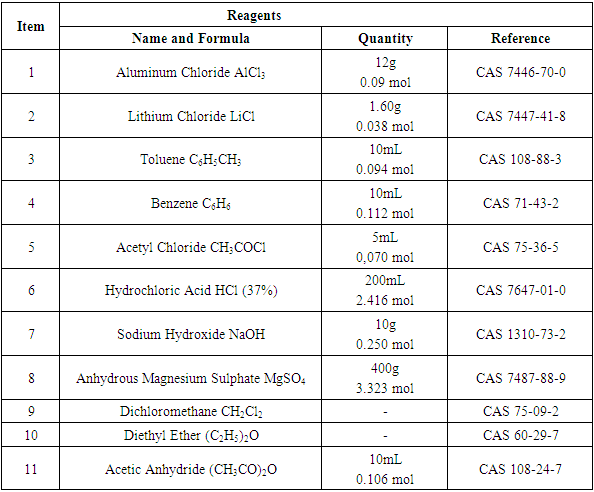 |
| |
|
2.1.2. Materials, Table 2
Table 2. F-C Reaction, Materials
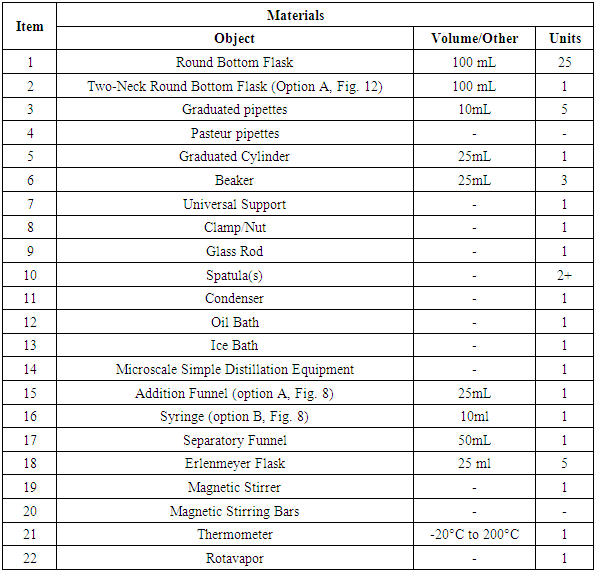 |
| |
|
2.2. Experimental Procedure Section
2.2.1. Acetophenone Synthesis (1)
The flask/reflux/shaker/bath system was assembled as shown in Figure 8. A mixture of aluminum chloride (1.30 g, 0.0750 mol) and benzene (1.85 mL, 0.0207 mol) was prepared in the flask (Figure 7 A, B). Acetic anhydride (3.92 mL, 0.0415 mol) was added to the flask slowly (dropwise) over period of 30 min, via addition funnel or syringe (Figure 8 A, B). It was heated under reflux for one hour. The reaction mixture turned a deep orange/brown color during the addition of acetic anhydride. It was allowed to cool to room temperature and it was added to a mixture of 45 ml of concentrated hydrochloric acid and 45 g of ice. After all of the reaction mixture had been poured, it was stirred for an additional 15 minutes. The mixture was then transferred to a separatory funnel with 25 ml of diethyl ether and the organic and aqueous phases were separated. The aqueous phase was extracted with another 25 ml portion of diethyl ether. The combined organic layer was washed with two 25 mL portions of 10% sodium hydroxide solution. The combined organic phase was separated and dried over magnesium sulphate. The dry organic phase was distilled in a simple distillation equipment under vacuum, a micro-scale equipment is suggested. The product obtained was acetophenone (1, 0.86 g, 34.4% yield).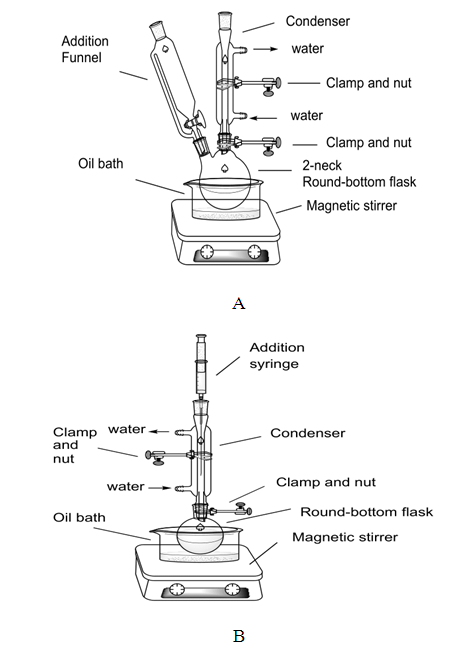 | Figure 7. Synthesis of Acetophenone (1), Two Equipment Options A or B |
2.2.2. 4–Methylacetophenone Synthesis (2) [8]
Set the experimental equipment of Fig. 8.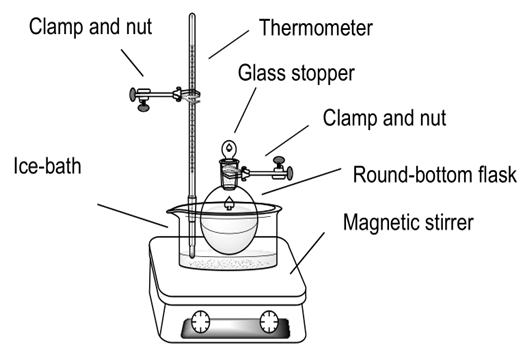 | Figure 8. Synthesis of p-Methylacetophenone (2) |
A mixture of aluminum chloride (10.00 g, 0.0750 mol) and lithium chloride (1.59 g, 0.0375 mol) in dichloromethane (15.00 mL) was prepared at -15°C. Toluene (2.66 mL, 0.0250 mol) and acetyl chloride (1.78 mL, 0.0250 mol) were added. The reaction mixture was left at -15°C for one hour (see Fig. 9), allowed to stand at room temperature overnight. 100 ml of a mixture of ice and dilute hydrochloric acid (concentration of 20%.) was slowly added to the reaction mixture, the organic phase was separated and the aqueous phase was extracted with two 50 ml aliquots of diethyl ether. The combined organic phases were then washed with 50 ml of dilute sodium hydroxide solution (concentration of 10%) and then with water, separated and dried over anhydrous magnesium sulphate. The dried solution was filtered to remove the drying agent and the solvents removed on a rotary evaporator. This ends manipulations. The product obtained was p-methylacetophenone (2, 2.86 g, 885.3%).
2.2.3. GC-MS Analyses and IR Spectrometry
The Gas chromatography coupled to EI mass spectroscopy (GC-MS) analyses were performed in a Shimadzu QCMS-QP2020 instrument, with a 30 m long Rxi ®-5Sil MS RETEK (Centre County, PA, U.S.) capillary column. 0.25 mm i.d. and helium carrier with flow rate of 1.0 mL/min; the temperature was programmed to be maintained at 60°C for 1 min and then increased at a rate of 5°C/min for 15 min and ended with 2 min at 250°C. Thus, the total ion chromatograms and mass spectra for acetophenone (1, Figure 9) and p-methylacetophenone (2, Figure 10) were obtained. 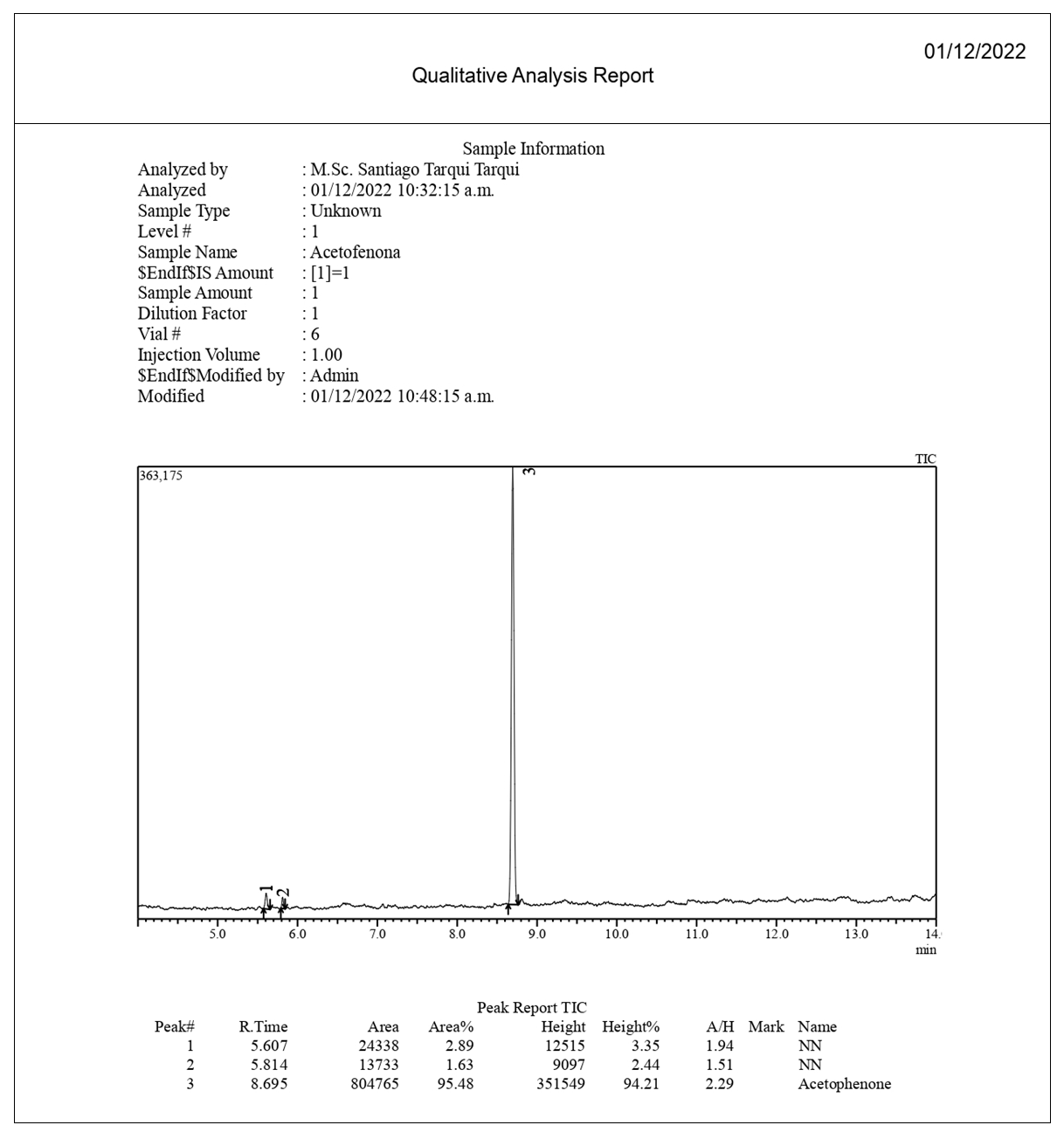 | Figure 9. GC-MS Acetophenone (1) Chromatogram |
 | Figure 9. Cont. GC-MS Acetophenone (1), Method |
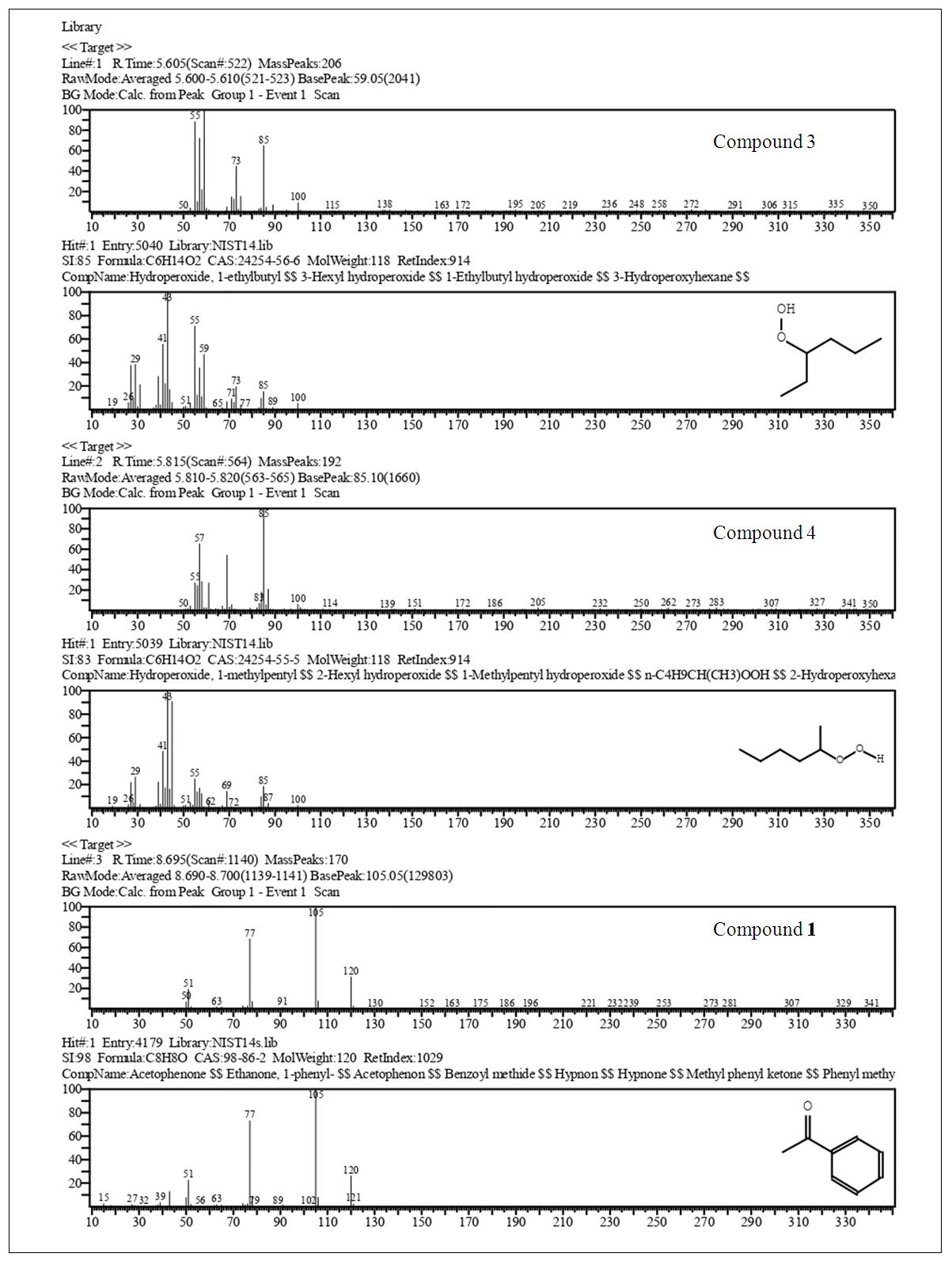 | Figure 9. Cont. GC-MS Acetophenone (1), EIMS Spectra of Compounds. Top: Experimental, Bottom: Library |
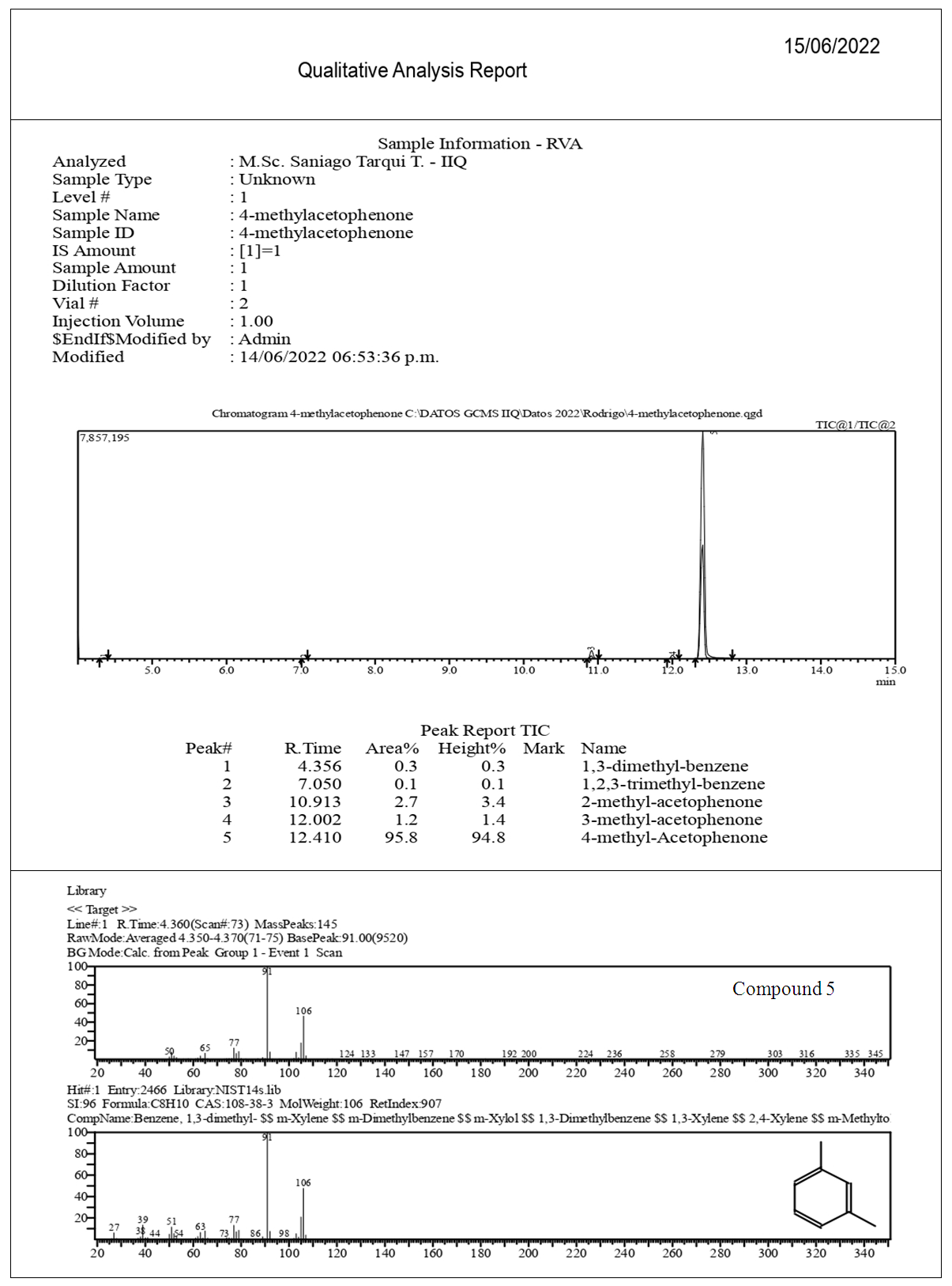 | Figure 10. GC-MS 4-Methylacetophenone (2), Chromatogram, EIMS Spectra of Compounds, Top: Experimental, Bottom: Library |
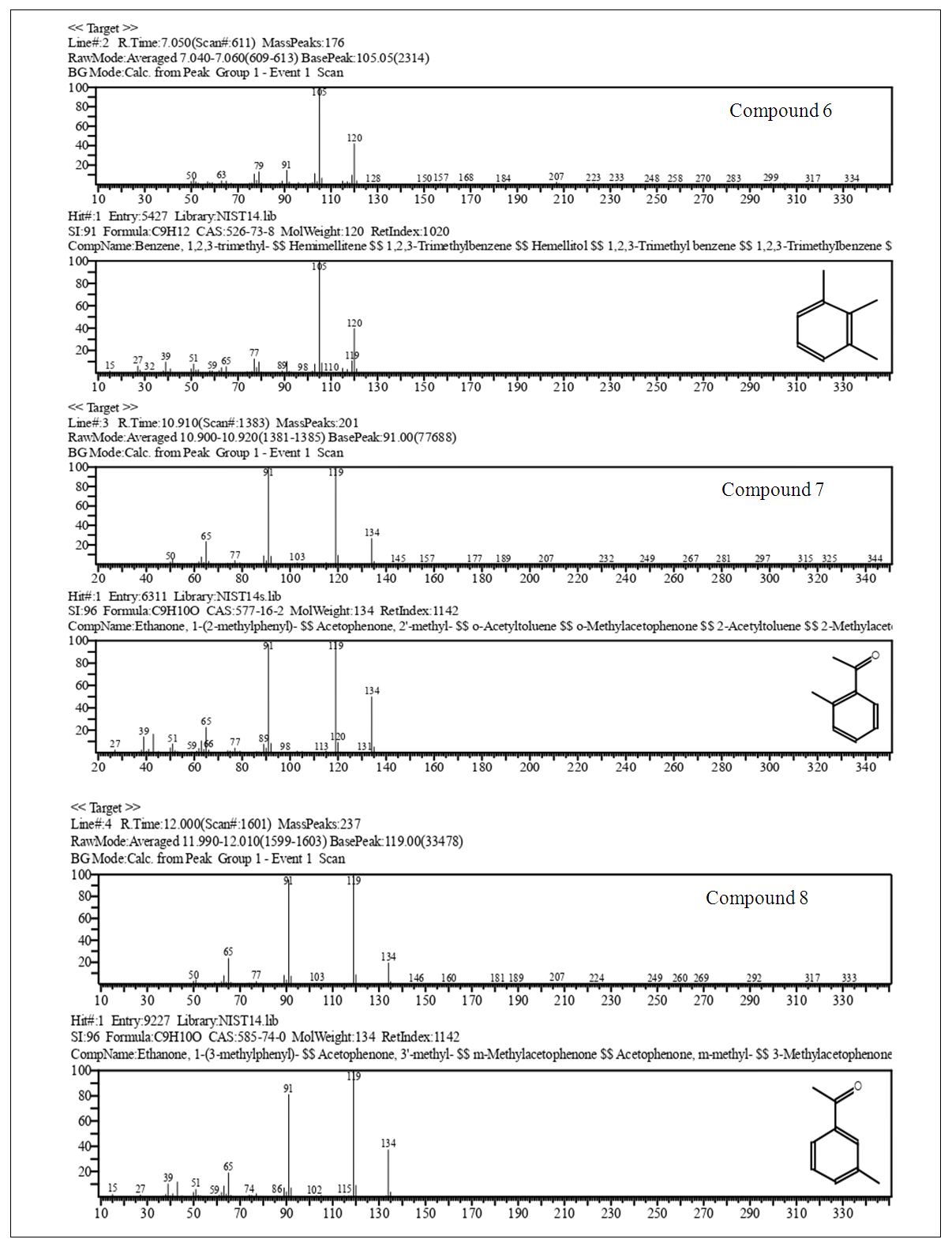 | Figure 10. Cont. GC-MS 4-Methylacetophenone (2), EIMS Spectra of Compounds, Top: Experimental, Bottom: Library |
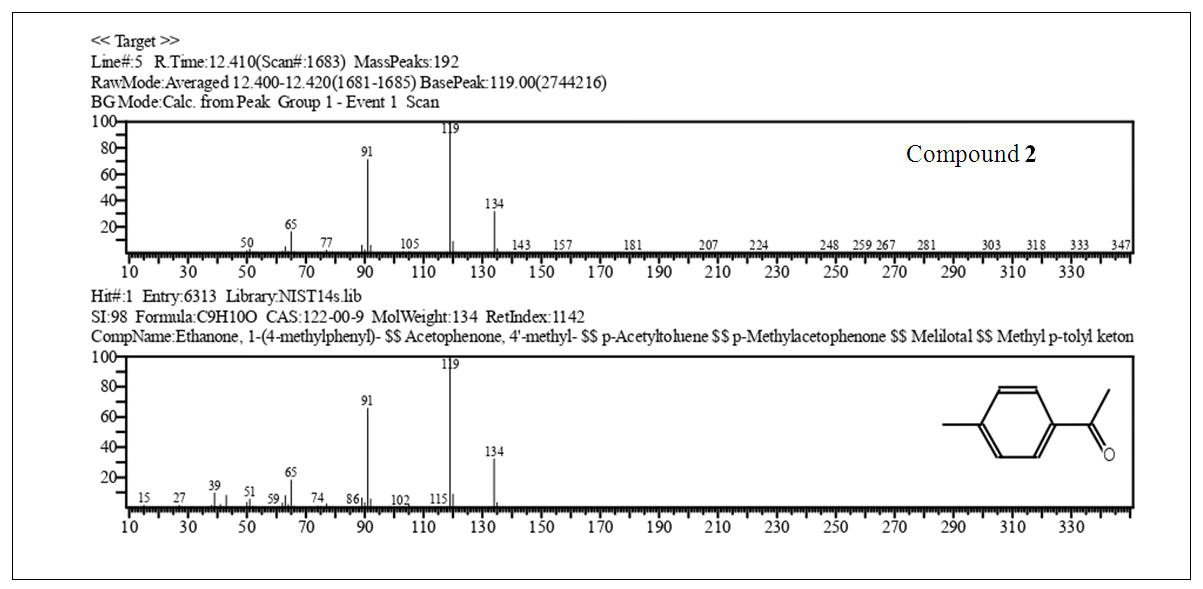 | Figure 10. Cont. GC-MS 4-Methylacetophenone (2), EIMS Spectra of Compounds, Top: Experimental, Bottom: Library |
2.2.4. IR Analyses of Acetophenone (1) and 4-Methylacetophenone (2)
IR apparatus was Perkin-Elmer Spectrum 1000, FTIR-IR and NEAR-IR spectroscopy using ZnSe support. 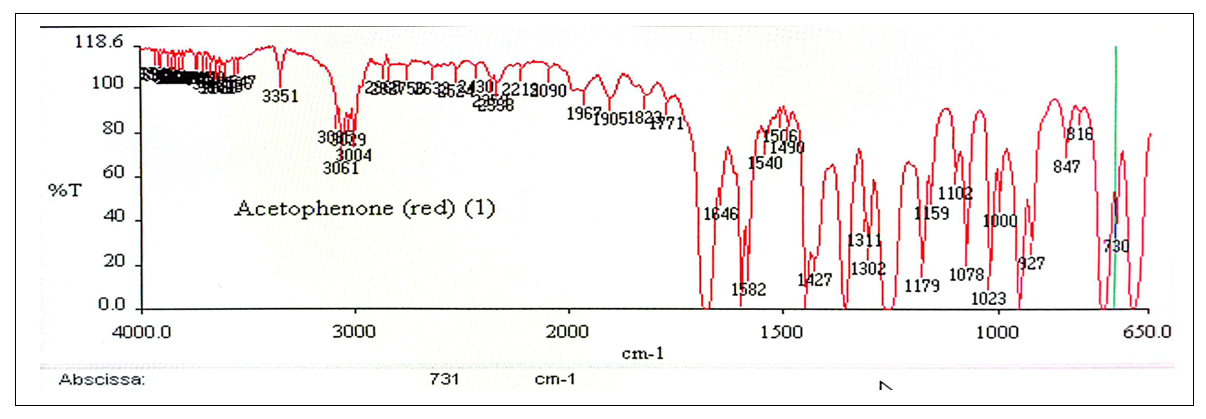 | Figure 11. IR Spectrum of Acetophenone (1) |
2.2.5. Comments on the Experimental Procedure Section
Friedel-Crafts acylation does not always give acceptable yields due to a number of factors; we proceed to a brief analysis of them below. It is important to mention that the Friedel-Crafts reaction mechanism (Figures 12 and 13) in the case of acid anhydrides is the same as that of acyl chlorides, but the aluminum complex formed would be aluminum trichloride acetate [9].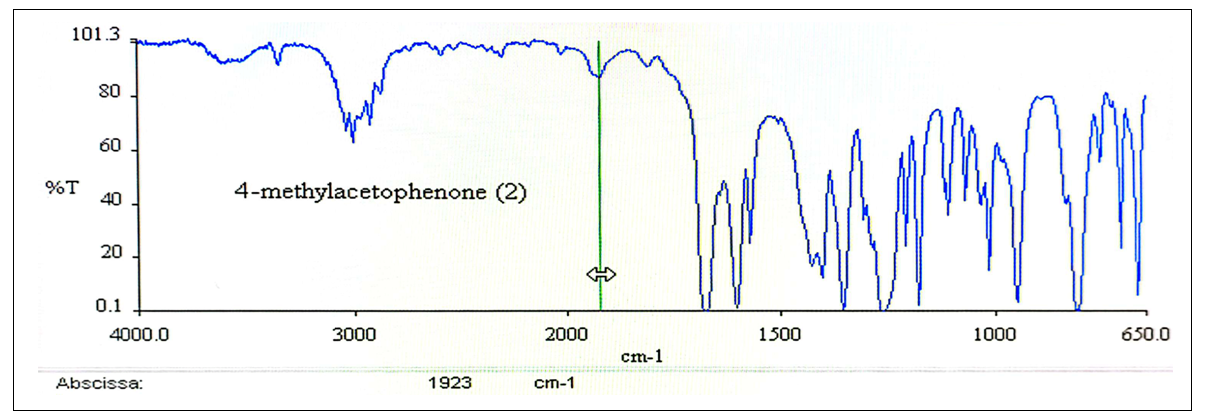 | Figure 12. IR Spectrum of 4-Methylacetophenone (2) |
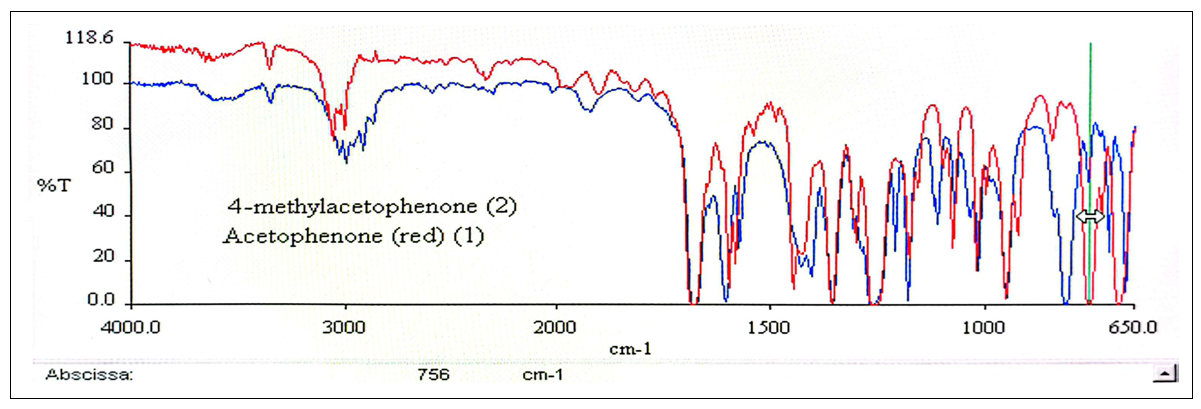 | Figure 13. IR Spectra, Comparative Overlapped View of 1 and 2 |
Polar solvents (nitrobenzene, nitroalkanes) are used for acylation in a homogeneous phase, conversely, non-polar solvents (dichloromethane, carbon disulfide, petroleum ether, carbon tetrachloride), for acylation in heterogeneous phase. A frequent practice is to use as solvent an excess of the aromatic compound to be substituted. The main influence of the solvent is manifested on the yield of the reaction, although it also influences the orientation of the substitution, fundamentally in polycyclic aromatics [10].Slight variations in temperature cause the formation of oils or resinous materials (cf. Introduction), reducing performance. It has also been found that an increase in temperature usually produces variations in the distribution percentages of the possible isomers.Aluminum trichloride is the most widely used catalyst in the Friedel-Crafts reaction due to its low cost and high catalytic power. However, other catalysts are also used such as metals, mineral acids, other metal halides, etc. Various series of transition metal halides have been examined and their catalytic power has been evaluated, with the maximum value generally corresponding to aluminum halides and particularly to chloride [11]. Among chlorides, the order of catalytic activity is: AlCl3 > SbCl5 > FeCl3 > TeCl2 > SnCl4 > TiCl4 > TeCl4 > BiCl3 > ZnCl2These effects can generally be minimized in the presence of solvents or reagents that complex aluminum trichloride such as lithium chloride [12]. The degree of purity of the aluminum trichloride is a particularly important factor and it is advisable to use freshly sublimated and anhydrous aluminum trichloride (Although anhydrous aluminum chloride is necessary to obtain high yields, it is not recommended to do this process for an undergraduate practice, as it is time consuming and expensive).Friedel-Crafts acylation and alkylation are homologous but not identical processes. The F-C alkylation requires relatively small (catalytic) amounts of aluminum chloride. On the other hand, at least one molar equivalent of aluminum chloride is needed for each carbonyl group present in the acylating agent in acylation. The Friedel-Crafts acylation is free of two features that complicate the alkylation reaction, (i) polysubstitution and (ii) rearrangements of the carbocations formed. There is usually no difficulty in stopping acylation with the introduction of a single acyl group into the aromatic nucleus, since the acyl group deactivates the nucleus and prevents further electrophilic attack.
2.2.6. Physicochemical Data of Compounds
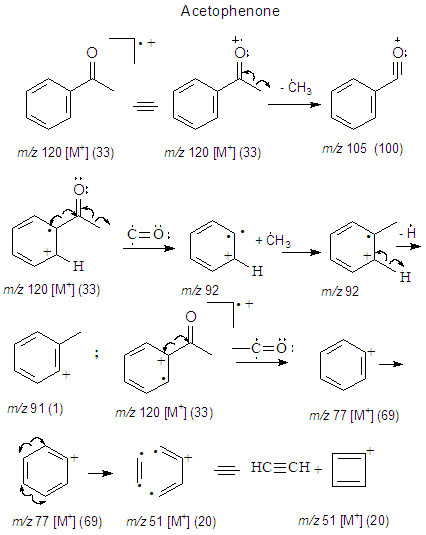
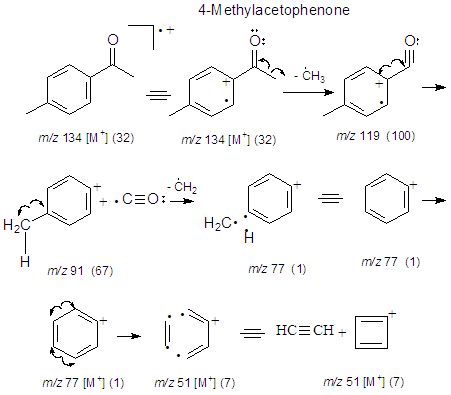
Major CompoundsCompound 1. Acetophenone. GC Ret time: 8.695 min; EIMS 70 eV: m/z (rel. int.): 120 [M]+ (33), 105 (100), 91 (1), 77 (69), 63 (2), 51 (20), 50 (8); IR (cm-1)  3351 (Overtone of C=O Stretch), 1646 (C=O Stretch), 1596, 1452, 1355, 1258, 936, 761 [13].Compound 2. .4-Methylacetophenone. GC Ret time: 12.410 min; EIMS 70 eV: m/z (rel. int.): 134 [M]+ (32), 119 (100), 91 (67), 65 (19), 51 (7). IR (cm-1) umax 3357 (Overtone of C=O Stretch), 1675 (C=O Stretch), 1607, 1428, 1357, 1268, 948, 790 [13].Minor CompoundsCompound 3. 3-Hydroperoxyhexane. GC Ret time: 5.607 min; EIMS 70 eV: m/z (rel. int.): 118 [M]+ (0), 100 (9), 85 (66), 73 (46), 59 (100), 57 (71), 55 (89), 50 (1).Compound 4. 2-Hydroperoxyhexane. GC Ret time: 5.814 min; EIMS 70 eV: m/z (rel. int.): 118 [M]+ (0), 100 (7), 85 (100), 69 (54), 61 (29), 57 (66), 55 (29), 50 (1).Compound 5. 1,3-Dimethylbenzene. GC Ret time: 4.356 min; EIMS 70 eV: m/z (rel. int.): 106 [M]+ (48), 91 (100), 77 (13), 65 (12), 50 (7).Compound 6. 1,2,3-Trimethylbenzene. GC Ret time: 7.050 min; EIMS 70 eV: m/z (rel. int.): 120 [M]+ (42), 119 (10), 105 (100), 91 (14), 79 (14), 63 (4), 50 (4).Compound 7. 2-Methylacetophenone. GC Ret time: 10.913 min; EIMS 70 eV: m/z (rel. int.): 134 [M]+ (28), 120 (10), 119 (100), 103 (1), 91 (100), 77 (5), 65 (23), 50 (6).Compound 8. 3-Methylacetophenone. GC Ret time: 12.002 min; EIMS 70 eV: m/z (rel. int.): 134 [M]+ (20), 120 (10), 119 (100), 103 (1), 91 (100), 77 (4), 65 (22), 50 (6).
3351 (Overtone of C=O Stretch), 1646 (C=O Stretch), 1596, 1452, 1355, 1258, 936, 761 [13].Compound 2. .4-Methylacetophenone. GC Ret time: 12.410 min; EIMS 70 eV: m/z (rel. int.): 134 [M]+ (32), 119 (100), 91 (67), 65 (19), 51 (7). IR (cm-1) umax 3357 (Overtone of C=O Stretch), 1675 (C=O Stretch), 1607, 1428, 1357, 1268, 948, 790 [13].Minor CompoundsCompound 3. 3-Hydroperoxyhexane. GC Ret time: 5.607 min; EIMS 70 eV: m/z (rel. int.): 118 [M]+ (0), 100 (9), 85 (66), 73 (46), 59 (100), 57 (71), 55 (89), 50 (1).Compound 4. 2-Hydroperoxyhexane. GC Ret time: 5.814 min; EIMS 70 eV: m/z (rel. int.): 118 [M]+ (0), 100 (7), 85 (100), 69 (54), 61 (29), 57 (66), 55 (29), 50 (1).Compound 5. 1,3-Dimethylbenzene. GC Ret time: 4.356 min; EIMS 70 eV: m/z (rel. int.): 106 [M]+ (48), 91 (100), 77 (13), 65 (12), 50 (7).Compound 6. 1,2,3-Trimethylbenzene. GC Ret time: 7.050 min; EIMS 70 eV: m/z (rel. int.): 120 [M]+ (42), 119 (10), 105 (100), 91 (14), 79 (14), 63 (4), 50 (4).Compound 7. 2-Methylacetophenone. GC Ret time: 10.913 min; EIMS 70 eV: m/z (rel. int.): 134 [M]+ (28), 120 (10), 119 (100), 103 (1), 91 (100), 77 (5), 65 (23), 50 (6).Compound 8. 3-Methylacetophenone. GC Ret time: 12.002 min; EIMS 70 eV: m/z (rel. int.): 134 [M]+ (20), 120 (10), 119 (100), 103 (1), 91 (100), 77 (4), 65 (22), 50 (6).
2.2.7. Hazards
All reagents comport a degree of toxicity when handling. The student must check out the hazards in the references of Table 1 (CAS number).
2.2.8. Tasks for the Student
Prepare a written report that includes:1. A flow chart of the manipulations2. A table with products, their yields and their degree of purity3. Mention of the isolation and purification procedures4. Notable comments on possible difficulties in handling5. New information on the Friedel-Crafts reaction and its industrial applications
3. Results and Discussion
3.1. Synthesis of 1 (Acetophenone)
The synthesis of 1 gave a low yield of 34% after the final distillation. This low yield can be attributed to the fact that the aluminum chloride was not completely dried for this procedure since its drying is a complex and tedious process which is not convenient for an undergraduate laboratory practice. Despite the low yield, gas chromatography (Figure 9) shows that acetophenone was obtained with high purity. The first two peaks (RT = 5.61 and 5.81 min) were identified as 3-Hydroperoxyhexane and 2-Hydroperoxyhexane (compounds 3 and 4 respetively by GC-MS (see section 2.2.6). Together they have a contribution to the product of 4.5%. Hydroperoxy function (Compounds 3 and 4) makes suspicion of EIMS rearrangements during run of spectra a fact signalled by T. Lund [14]. On the other hand, the third peak (RT = 8.695 min) corresponds to 1 with a contribution of 95.5%. In this case, despite the fact of a low yield the product obtained has a high purity being suitable for subsequent synthesis.
3.2. Synthesis of 2 (4-Methylacetophenone)
The synthesis of 2 gave a good yield (85%) after the final distillation. In order to verify the purity of the product obtained it was analyzed by gas chromatography -mass spectrometry (GC-MS, Fig. 10). The chromatogram shows five compounds. Compounds 5, 1,3-Dimethylbenzene, and compound 6, 1,2,3-Trimethylbenzene (see section 2.2.6.). These compounds are minor products of the synthesis F-C. For instance, 1,2,3-methylbenzene also called hemimellitene (CAS [526-73-8]) is industrially produced during petrol distillation in the C9 fraction of aromatic hydrocarbons; also, Trimethylbenzenes are products from methylation of toluene or xylene according to Friedel – Crafts reaction when chloromethane in the presence of aluminum chloride is employed [15]. Compounds 7, 8 and 2 correspond after the GC-MS analysis to three isomers, 2-, 3- and 4-acetophenone, respectively. Compound 7 (RT 10.913) with a contribution to the product of 2.7%, compound 8 (RT 10.002) with a contribution of 1.2% and compound 2 (RT 12.410) 95.8%. The elution order in GC of the three isomers (cmpds 7, 8 and 2) is, according to the recorded retention times, 7, first, 8, second, and the last one is 2. However, when an equimolar mixture of these three compounds is separated by GC, the most volatile of the three (lowest boiling point) elutes first (lowest retention time), and sequentially after elute the other two with higher boiling points, namely the order is 2 (4-MeAcOPhenone, 226°C, 1st), 8 (3-MeAcOPhenone, 234°C, 2nd) and 7 (2-MeAcOPhenone, 242°C, 3rd).Boiling points4-Methylacetophenone (p-Methylacetophenone): 226°C. (2)3-Methylacetophenone (m-Methylacetophenone): 234°C. (8)2-Methylacetophenone (o-Methylacetophenone): 242°C. (7)This poses the reverse order with respect to the results obtained. We can explain this if we consider that the products of the three isomers after a Friedel-Crafts acylation aren’t in equimolar proportions. Actually, the percentages according to Figure 10 are 7 (ortho-methyl isomer, RT 10.913, 2.7%), 8 (meta-methyl isomer, RT 10.002, 1.2%) and 2 (para-methyl isomer, RT 12.410, 95.8%). Thus, the less abundant component of the mixture should boil first (8, 1.2%), then the following in increasing mass (7, 2.7%), and finally the most abundant (2, 95.8%). What we observe here is two driving forces for boiling-elution. The first one is the boiling point, and the second is the mass of each component. One of them predominates over the other, temperature vs. mass. It is clear according to the results (chromatogram of Figure 10), that 2 elutes last, here mass predominates over boiling point (T°). For the minor components 7 and 8, the fact that 8 (lower relative boiling point) weighs more than 7, precludes the influence of 7’s higher boiling point. Thus 7 elutes before 8. The next important aspect to be discussed is why in this acylation (toluene’s acylation) the para-isomer product is favored over the other two isomers, ortho and meta, and also why meta overshadows ortho. The answers will emerge of the focus on the mechanisms, exposed in the next section.
3.3. Friedel-Crafts Acylation Mechanisms
The F-C method allows the direct union of an acyl group to an aromatic ring and is called Friedel-Crafts acylation. In this reaction, benzene (or derivative) undergoes electrophilic aromatic substitution (EAS) when treated with an acylating agent, acyl halide or acid anhydride in the presence of a Lewis acid catalyst. Two possible mechanisms [9].
3.3.1. Mechanism for Acetophenone. Acylation of Benzene, See Figure 14 [9]
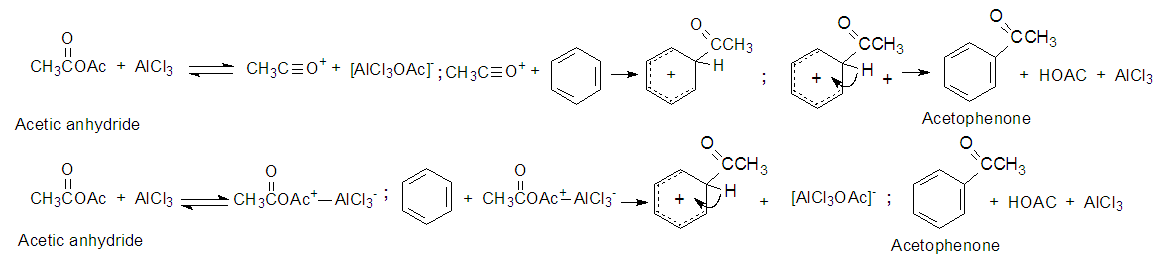 | Figure 14. Friedel Crafts Acylation of benzene and acetyl chloride to form acetophenone |
3.3.2. Mechanism for 4-Methylacetophenone. Acylation of toluene, see Figure 15 [9,16]
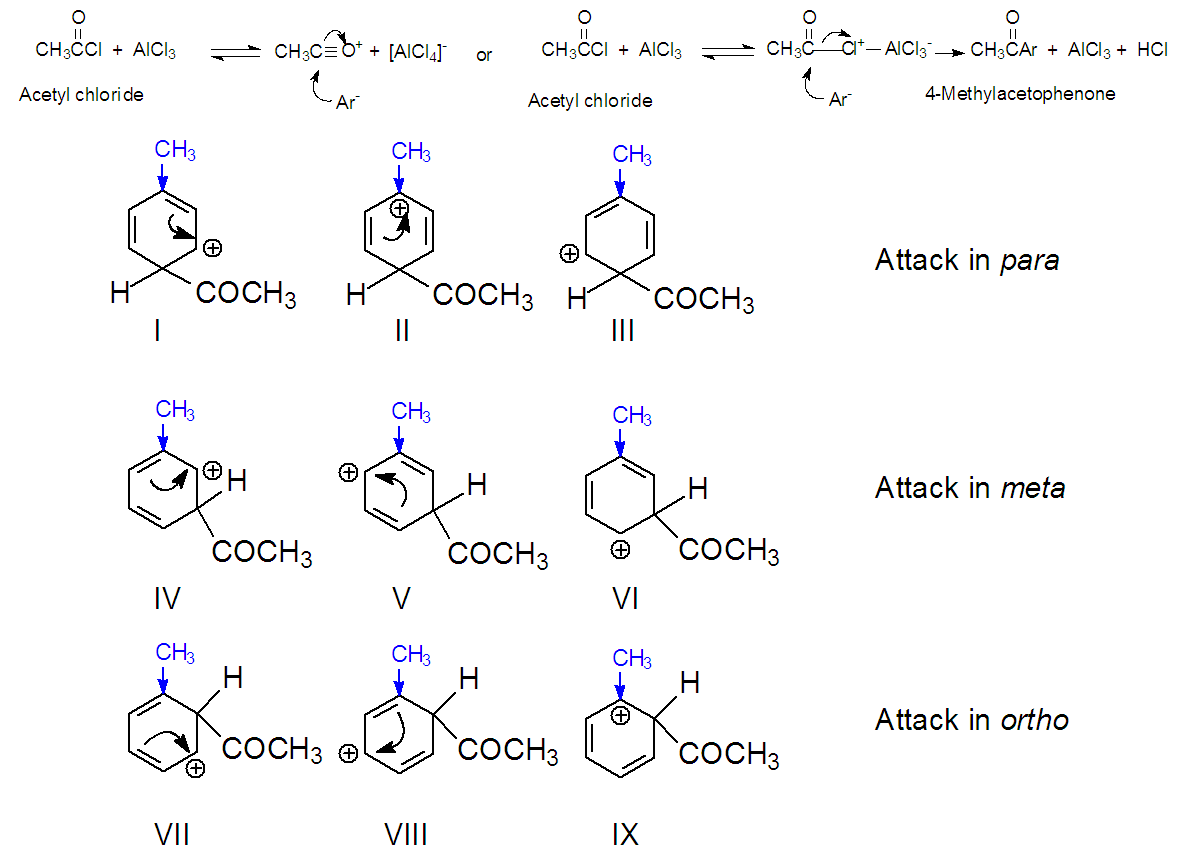 | Figure 15. Mechanism of Acylation of Toluene to form 4-Methylacetophenone (2) [9,16] |
The activating group methyl of toluene, activates all positions of benzene, and it’s an ortho/para director because these positions become more activated than the meta position. Figure 15 shows the same mechanisms exposed for acetophenone. The methyl group in toluene directs a preferential attack on Ar- on the ortho/para positions. Let us compare, for example, the carbocations generated by the attack on positions para and meta of toluene. Each cation is a hybrid of three structures I-III for para and IV to VI for meta. In structure II, the positive charge is located on the carbon supporting the methyl group. Even though this one liberates electrons for all positions in the ring, its charge in electrons is preferentially addressed to the closest carbon. Hence structure II is particularly stable. Due to this contribution of structure II, the hybrid carbocation resulting from an attack in position para is more stable that the one afforded by an attack in meta ( IV–VI). Hence, substitution is faster on para than meta. The same feature can be observed when the attack in on ortho (VII-IX), here, also a more stable carbocation is generated, because of the contribution of IX, in comparison to the meta attack [16]. Thus, this mechanism supports the obtained relative amounts of the three isomers of methyl acetophenone by F- C. The much faster attack on the para position, overwhelms the attacks on the other positions. The ortho position attack is slower than para in spite of having the same hybrid carbocation. The reason is steric hindrance generated by the proximity of both substituents in the ring, methyl and acetyl.
3.4. Identification of Products
All major and minor products of the F-C acylation were characterized, 8 products in all (see section 2.2.6). The main tool was Gas Chromatography-Mass Spectrometry (GC.MS). An easily profitable and easy doing technique for fast results in quantification and identification, particularly as complement in synthesis works by undergraduate students. Figures 9 and 10 show the chromatograms and mass spectra for both synthesis products. The target products of the syntheses were compound 1 (acetophenone) and compound 2 (p-methylacetophenone), both major products of these experiments. The technique gives very clearly the identification of all products based on the retention times and EIMS spectra. Hence, no ambiguity in characterization is possible. We’ll expose the EIMS fragmentation pattern of the major products, main goal of this lab guide, compounds 1 and 2. As complementary identification tool, the IR spectra of 1 and 2 were run.
3.4.1. EIMS’ Fragmentation Pattern of Acetophenone (1)
3.4.2. EIMS’ Fragmentation Pattern of 4-Methylaceto- Phenone (2)
4. Conclusions
A practical guide for organic chemistry laboratory was proposed on the subject of synthesis with the preparation of two aromatic ketones using the acylation method according to Friedel-Crafts with real and verifiable results. The identification of the syntheses products was carried out with the GC-MS technique due to its lower costs compared to a NMR analysis. The exposition of the manipulation was done in a detailed manner with a supplementary character beyond what is found as bibliography available to undergraduate chemistry students. For this, this guide deals with basic synthetic tools such as Friedel-Crafts acylation simple operation to be mastered for future even more interesting synthetic forays. Given the fact of high altitude of our laboratory (3600 m.a.s.l., about 486.9 mmHg of atmospheric pressure) some synthetic experiments can show lower yield in comparison to values reported from literature of the homologue treatment at sea level. This could be a cause for the poor yield of acetophenone (1) by F-C. The Organic Chemistry Notebook Series, a Didactical Approach pretends, besides proposing mechanistic views, to vow simple and accessible practices making use of starting materials and reagents that are common in the organic chemistry laboratory. Some other didactical approaches on the F-C acylation devoted to students in the lab have been surveyed as bibliography precedingly, and have inspired and guided somehow the present job. It is worth mentioning A. M. Reeve, that proposed A Discovery-Based Friedel–Crafts Acylation Experiment: Student-Designed Experimental Procedure [17]. In this regard, two of the authors, J. J. D. and A. C. are currently undergraduate and graduate students, respectively in our Chemical Research Institute. Another interesting paper deals with the structure determinations of the final products of the F-C acylation, by employing IR spectrometry [18]. A more recent approach to the F-C acylation was the employing of MW synthesis with Microwave-Assisted Friedel-Crafts Acylation of Toluene with Anhydrides. Compounds were characterized by 1H NMR, 13C NMR and DEPT pulses [19]. Thus, to the best of our knowledge, the present guide is innovating regarding the identification of products with a more modern technique, that presents multiple advantages regarding previous works on educational themes like synthesis of simple compounds like Friedel-Crafts Acylation.
ACKNOWLEDGEMENTS
The authors express their gratitude to Mr. Santiago Tarqui, M.Sc., research technician at the Laboratory of Analytical Services of the Chemical Research Institute IIQ-UMSA for performing the GC-MS analyses. The authors thank the Chemical Research Institute IIQ-UMSA for its institutional and financial support, and Dr. Michel Sauvain from the Institut de Recherche pour le Développement of France for financial support.
References
| [1] | C. Friedel and J. M. Crafts, 1877, Sur une Méthode Générale Nouvelle de Synthèse d’Hydrocarbures, d’Acétones, etc., Compt. Rendus, 84 1392-1395. |
| [2] | C. Friedel and J. M. Crafts, 1877, Sur une Méthode Générale Nouvelle de Synthèse d’Hydrocarbures, d’Acétones, etc., Compt. Rendus, 84 1450-1454. |
| [3] | C. Friedel and J. M. Crafts, 1877, Sur une Méthode Générale Nouvelle de Synthèse d’Hydrocarbures, d’Acétones, etc., Compt. Rendus, 85 74-77. |
| [4] | J. Wisniak, 2009, Para quitarle el polvo, Charles Friedel, Educación Química, 20 (4) 447-455. https://doi.org/10.22201/fq.18708404e.2009.4. |
| [5] | C. Friedel and J. M. Crafts, 1888, Sur une Nouvelle Méthode Générale de Synthèse des Combinaisons Aromatiques, Ann. Chim. Phys. [6] 14 433-472. |
| [6] | C. Friedel and J. M. Crafts, 1874, Bromo-Iodure d’Éthylène, Bull. Soc. Chim. 21 435. |
| [7] | C. Friedel and J. M. Crafts, 1884, Sur une Nouvelle Méthode Générale de Synthèse des Combinaisons Aromatiques, Ann. Chim. Phys. [6] 1 449-532. |
| [8] | H. C. Gors, P. J. Horner, V. Jansons, Friedel-crafts preparation of aromatic ketones with an inorganic salt controlling agent. 1991, Google Patents. |
| [9] | F. A. Carey, and R. J. Sundberg, 1990, Advanced Organic Chemistry: Part A: Structure and Mechanisms, Plenum Press, New York. |
| [10] | P. H. Gore, 1955, The Friedel-Crafts Acylation Reaction and its Application to Polycyclic Aromatic Hydrocarbons. Chemical Reviews, 55(2) 229-281. https://doi.org/10.1021/cr50002a001. |
| [11] | G. A. Olah, 1963, Friedel-Crafts and Related Reactions, Interscience Publishers, New York. |
| [12] | H. C. Brown, B. A. Bolto and F. R. Jensen, 1958, Relative Rates of the Aluminum Chloride-Catalyzed Benzoylation of the Monoalkylbenzenes in Nitrobenzene Solution. Importance of Carbon-to-Carbon Hyperconjugation in Alkyl Substituents 1,2, The Journal of Organic Chemistry. 23(3) 414-416. https://doi.org/10.1021/jo01097a024. |
| [13] | R. M. Silverstein., G. C. Bassler, T. C. Morill, 1991, Spectrometric Identification of Organic Compounds, John Wiley & Sons, Inc., New York. |
| [14] | T. Lund, Lecture notes in EI-Mass spectrometry By Torben Lund RUC 2015, 2015, https Link: Lecture notes in Mass spectrometry: Fragmentation mechanisms in electron impact mass spectrometry. |
| [15] | K. Griesbaum, A. Behr, D. Biedenkapp, H.-W. Voges, D. Garbe, C. Paetz, G. Collin, D. Mayer, H. Höke, Hydrocarbons" in Ullmann's Encyclopedia of Industrial Chemistry 2002 Wiley-VCH, Weinheim. https://doi.org/10.1002/14356007.a13_227. |
| [16] | R. T. Morrison, R. N. Boyd, 1990, Organic Chemistry, Addison-Wesley, Willmington. |
| [17] | A. McElwee Reeve, 2004, A Discovery-Based Friedel–Crafts Acylation Experiment: Student-Designed Experimental Procedure, J. Chem. Educ., 81 10 1497. https://doi.org/10.1021/ed081p1497. |
| [18] | P. F. Schatz, 1979, Friedel-Crafts Acylation, An experiment Incorporating Spectroscopic Structure Determination, J. Chem. Educ., 56 (7), 480. https://doi.org/10.1021/ed056p0. |
| [19] | M. Dallas, M. Wagner, 2013, A Microwave-Assisted Friedel–Crafts Acylation of Toluene with Anhydrides, J. Chem. Educ. 90 (3) 390–392. https://doi.org/10.1021/ed200479n. |







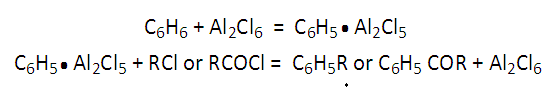











 3351 (Overtone of C=O Stretch), 1646 (C=O Stretch), 1596, 1452, 1355, 1258, 936, 761 [13].Compound 2. .4-Methylacetophenone. GC Ret time: 12.410 min; EIMS 70 eV: m/z (rel. int.): 134 [M]+ (32), 119 (100), 91 (67), 65 (19), 51 (7). IR (cm-1) umax 3357 (Overtone of C=O Stretch), 1675 (C=O Stretch), 1607, 1428, 1357, 1268, 948, 790 [13].Minor CompoundsCompound 3. 3-Hydroperoxyhexane. GC Ret time: 5.607 min; EIMS 70 eV: m/z (rel. int.): 118 [M]+ (0), 100 (9), 85 (66), 73 (46), 59 (100), 57 (71), 55 (89), 50 (1).Compound 4. 2-Hydroperoxyhexane. GC Ret time: 5.814 min; EIMS 70 eV: m/z (rel. int.): 118 [M]+ (0), 100 (7), 85 (100), 69 (54), 61 (29), 57 (66), 55 (29), 50 (1).Compound 5. 1,3-Dimethylbenzene. GC Ret time: 4.356 min; EIMS 70 eV: m/z (rel. int.): 106 [M]+ (48), 91 (100), 77 (13), 65 (12), 50 (7).Compound 6. 1,2,3-Trimethylbenzene. GC Ret time: 7.050 min; EIMS 70 eV: m/z (rel. int.): 120 [M]+ (42), 119 (10), 105 (100), 91 (14), 79 (14), 63 (4), 50 (4).Compound 7. 2-Methylacetophenone. GC Ret time: 10.913 min; EIMS 70 eV: m/z (rel. int.): 134 [M]+ (28), 120 (10), 119 (100), 103 (1), 91 (100), 77 (5), 65 (23), 50 (6).Compound 8. 3-Methylacetophenone. GC Ret time: 12.002 min; EIMS 70 eV: m/z (rel. int.): 134 [M]+ (20), 120 (10), 119 (100), 103 (1), 91 (100), 77 (4), 65 (22), 50 (6).
3351 (Overtone of C=O Stretch), 1646 (C=O Stretch), 1596, 1452, 1355, 1258, 936, 761 [13].Compound 2. .4-Methylacetophenone. GC Ret time: 12.410 min; EIMS 70 eV: m/z (rel. int.): 134 [M]+ (32), 119 (100), 91 (67), 65 (19), 51 (7). IR (cm-1) umax 3357 (Overtone of C=O Stretch), 1675 (C=O Stretch), 1607, 1428, 1357, 1268, 948, 790 [13].Minor CompoundsCompound 3. 3-Hydroperoxyhexane. GC Ret time: 5.607 min; EIMS 70 eV: m/z (rel. int.): 118 [M]+ (0), 100 (9), 85 (66), 73 (46), 59 (100), 57 (71), 55 (89), 50 (1).Compound 4. 2-Hydroperoxyhexane. GC Ret time: 5.814 min; EIMS 70 eV: m/z (rel. int.): 118 [M]+ (0), 100 (7), 85 (100), 69 (54), 61 (29), 57 (66), 55 (29), 50 (1).Compound 5. 1,3-Dimethylbenzene. GC Ret time: 4.356 min; EIMS 70 eV: m/z (rel. int.): 106 [M]+ (48), 91 (100), 77 (13), 65 (12), 50 (7).Compound 6. 1,2,3-Trimethylbenzene. GC Ret time: 7.050 min; EIMS 70 eV: m/z (rel. int.): 120 [M]+ (42), 119 (10), 105 (100), 91 (14), 79 (14), 63 (4), 50 (4).Compound 7. 2-Methylacetophenone. GC Ret time: 10.913 min; EIMS 70 eV: m/z (rel. int.): 134 [M]+ (28), 120 (10), 119 (100), 103 (1), 91 (100), 77 (5), 65 (23), 50 (6).Compound 8. 3-Methylacetophenone. GC Ret time: 12.002 min; EIMS 70 eV: m/z (rel. int.): 134 [M]+ (20), 120 (10), 119 (100), 103 (1), 91 (100), 77 (4), 65 (22), 50 (6).


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML

