-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2024; 12(2): 36-43
doi:10.5923/j.jlce.20241202.02
Received: Nov. 7, 2024; Accepted: Dec. 12, 2024; Published: Dec. 21, 2024

Synthesis of Biodiesel as an Introduction to Education for Sustainable Development for High School Students: Beware of the (Surface-Active) Minor Oil Components!
Omar A. El Seoud 1, Maria Helena Zambelli 2, Raquel N. Guimarães 3, Kátia Regina V. Roa 4, Francisco Mateus Alves 5, Lize Silvério 6, Nicolas Keppeler 1, Narciso R. S. Vagula 1, João Pedro R. Marcacini 1, Naved I. Malek 7
1Institute of Chemistry, University of São Paulo, São Paulo, Brazil
2Humboldt College, São Paulo, Brazil
3Prof. Luiz Gonzaga Righini State High School, São Paulo, Brazil
4Prof. Mario M. D. de Aquino State High School, São Paulo, Brazil
5Major Telmo Coelho Filho State High School, São Paulo, Brazil
6Immaculate Mary College, São Paulo, Brazil
7S. V. National Institute of Technology, Surat, Gujarat, India
Correspondence to: Omar A. El Seoud , Institute of Chemistry, University of São Paulo, São Paulo, Brazil.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

As a contribution to the United Nations Educational, Scientific and Cultural Organization (UNESCO) Education for Sustainable Development program that includes UNESCO´s Climate Change Education program, we organized a project for the third (or last) year of high school students on the synthesis of biodiesel (BD) from babassu oil (BO) and soybean oil (SO). The project is composed of the following stages: before the visit, a questionnaire was given to the students regarding their knowledge of the theme BD. Subsequently, the students spent one day at the Institute of Chemistry of the University of São Paulo. During their visit (7 hours, including a lunch-time break) they synthesized BD samples of both oils; calculated the saponification- and iodine values of BO and SO, and were introduced to analysis by gas chromatography. Because of lack of laboratory time, the students submitted their (slightly turbid) BD samples; these were further dried by the instructors. The BD yields were then calculated by the students from the dynamic viscosities of their samples and calibration curves. The experiments with BO were problem-free, those using SO were problematic because of the formation of stable water/oil emulsions that required a long time to phase-separate. We attributed this emulsion formation to the presence of (surface active) phospholipids in SO. These minor components were removed by treatment of an ethanolic solution of SO with a basic ion-exchange resin at room temperature, followed by removing ethanol. The experiments with this treated SO were problem-free. In a post-visit assessment, the students evaluated this project as innovator, interesting, and motivating. This project is simple, safe, low cost and represents a “hands-on” introduction to green chemistry.
Keywords: Biodiesel Synthesis, Education for Sustainable Development, High School Students
Cite this paper: Omar A. El Seoud , Maria Helena Zambelli , Raquel N. Guimarães , Kátia Regina V. Roa , Francisco Mateus Alves , Lize Silvério , Nicolas Keppeler , Narciso R. S. Vagula , João Pedro R. Marcacini , Naved I. Malek , Synthesis of Biodiesel as an Introduction to Education for Sustainable Development for High School Students: Beware of the (Surface-Active) Minor Oil Components!, Journal of Laboratory Chemical Education, Vol. 12 No. 2, 2024, pp. 36-43. doi: 10.5923/j.jlce.20241202.02.
Article Outline
1. Introduction
- The United Nations Educational, Scientific and Cultural Organization (UNESCO) is leading the effort to implement the Decade of Education for Sustainable Development (ESD) for 2030 [1], and the Climate Change Education (CCE) program. The objective of the latter is to equip the populations to cope with-, and mitigate the effects of climate changes that are becoming painfully clear [2]. Worldwide, the CO2 emission produced by the transport sector represents ca. 23% of the total man-produced CO2 emission. There is a widespread agreement to reduce emissions from transport by a minimum of 50% by 2050, at the latest [3].Compared to diesel oil, the use of biodiesel (BD) from vegetable oils and fats results in the reduction of greenhouse emissions by 40% to 69% [4]. Other favorable properties of BD that support its use is that the B20 fuel (diesel that contains 20% BD) can be used without any engine modification [5]. Additionally, BD raises the cetane number (CN) of the fuel, thus decreases its “ignition delay”, i.e., the time between start of the ignition and the first pressure increase during combustion; this increases engine efficiency. The higher BD viscosity, relative to that of mineral diesel oil, improves lubricity, and reduces friction within the moving parts [6]. With these advantages in mind, many students are being introduced to the synthesis of BD from oils and fats, and determination of their properties as fuels [7]. At the Institute of Chemistry of the University of São Paulo (ChemUSP) we introduced this theme on the graduate level, and also to high school students. In our previous work we employed babassu oil (BO) that is produced in a relatively small quantity (26,475 tons in 2023; [8]) in the north- and north-eastern parts of Brazil. We decided to introduce soybean oil (SO), because Brazil is the third largest SO producer worldwide (10.8 million tons in 2023; [9]), and most of commercially produced BD in Brazil uses SO [10]. In this experiment, the vegetable oils are transesterified with ethanol (EtOH) in the presence of sodium ethoxide catalyst, followed by BD separation by washing. Because of the limited laboratory time at ChemUSP, the oil /water phase separation must be fast, i.e., occurring during minutes, not hours. Whereas the experiments with BO were problem-free, the first group of students that used SO ran into the problem of very slow phase separation that took many hours to occur. We attributed this emulsion stability to the presence of minor amounts of (surface active) phospholipids in SO. These were removed by pretreatment of SO with anionic ion-exchange resin. Our solution worked, and the students were able to use both oils. This problem was explained to the students who appreciated the underlying reason, and how chemistry solved it.
2. Experimental
2.1. Materials
- Commercial absolute ethanol (EtOH) 99.8%; ethylene glycol (EG); standardized HCl and NaOH solutions were from LabSynth, SP, and were used as received. Ambersep -900 OH anion-exchange resin was from Aldrich and was dried under reduced pressure, at 50°C until a constant mass. Babassu oil (BO) was from COPPALJ, Maranhão; soybean oil (SO; Liza brand; Cargill) was bought at a local supermarket. The content of free fatty acids of both oils was below the limit established by the Brazilian legislation [11]. The sodium ethoxide catalyst was freshly prepared before the experiment, by dissolving sodium metal in EtOH, using an Erlenmeyer flask protected with a drying tube. The concentration of the base was determined by titration with standardized HCl solution; the final base concentration was adjusted to 0.152 mol/L. The material necessary for the experiments includes: burettes of appropriate volumes; three-necked round-bottom reaction flasks; Erlenmeyer flasks; semi-analytical balances; hotplates with magnetic stirring; borosilicate glass cylindrical oil baths containing ethylene glycol (EG); red spirit-filled immersion thermometers, and separation funnels.
2.2. Procedures
2.2.1. Quantitative Transformation of Vegetable Oils into the Corresponding Biodiesel
- This experiment was carried out by the instructors before the visits. BO and SO were quantitatively transformed into the corresponding BDs by reacting each oil with a large excess of EtOH (alcohol: oil volume ratio = 30:1), using concentrated H2SO4 as a catalyst (catalyst: ethanolic oil solution = 10%, w/w) [12]. The completeness of the reaction was confirmed by CG/MS analysis using Shimadzu model CG msQP2010, equipped with BPX5 (5% phenyl methylpolysiloxane) - 30m capillary column, that was heated according to the following temperature program: 5 min at 120°C; increase to 240°C at 8°C/minute; 8 min at 240°C. These samples will be denoted BD-100%.
2.2.2. Pretreatment of the Soybean Oil
- This experiment was carried out by the instructors before the visits. In a stoppered Erlenmeyer flask, SO was mixed with half its volume of EtOH and the mixture was stirred with dry Ambersep-900 resin (1g resin/20 mL of SO) at room temperature, for 30 minutes. The resin was filtered off, and the ethanol was removed under reduced pressure.
2.2.3. Determination of the Saponification Values (SVs) of the Vegetable Oils
- Because of lack of time, the students received the oils already saponified by using an alcoholic KOH solution, as given elsewhere [13]. They back-titrated the excess KOH using phenolphthalein indicator, and calculated SV (given in mg KOH/g oil) by using Equation 1:
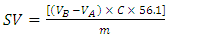 | (1) |
2.2.4. Determination of the Iodine Values (IVs) of the Vegetable Oils
- The IV is the mass of iodine, in g, consumed by 100 g of oil. It is determined by adding I2 solution to the oil, followed by titration of the excess halogen using a standardized Na2S2O3 solution. This experiment was carried out by the instructor as a demonstration during the visits. The ISO 3961 method was used, except that the iodine/thiosulfate titration was carried out potentiometrically, using Orion Star T920 Redox Titrator with platinum electrodes. The value of IV is calculated from Equation 2:
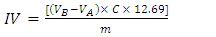 | (2) |
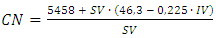 | (3) |
2.2.5. Synthesis of Biodiesel Samples
- This experiment was carried out by the students in groups, each of 2 or 3. Half of the groups used BO, the other half used pre-treated SO. The reaction conditions and the order of the experiments were generated by version 13.3 of the Statistica software (StatSoft, TIBCO Software).The instructions given to the students were as follows: Place the provided EG heating bath (containing a magnetic bar) on a hotplate/magnetic stirrer. Place the (hotplate + EG-bath) on a laboratory scissor jack. Insert the provided thermometer into the bath and begin the (stirring + heating) of the EG until its temperature reaches 100 ± 5°C. Concurrently, introduce (do not drop) a magnetic stirring bar into a 3-necked, 125 mL round-bottom flask; mark the flask with the groups´ name and the oil used (e.g., G1-BO, G2-SO, etc). Use the provided glass graduated cylinder to measure 20 mL of the vegetable oil. Using an addition funnel, introduce the oil into the reaction flask. Using a burette introduce the appropriate volume of EtOH, as indicated in Table 1 below. Once the oil and EtOH were introduced, close the three "necks" of the round-bottom flask with the provided polypropylene stoppers. Assemble the transesterification reactor above (not inside) the EG-bath, by inserting a reflux condenser provided with a CaCl2 tube (drying agent) in the central “neck” of the flask. When the temperature of the EG bath reaches the indicated range (100 ± 5°C), circulate water into the reflux condenser. Use a 10 mL burette to introduce the catalyst solution (C2H5ONa/C2H5OH; 0.152 mol/L), then use the laboratory jack to slowly raise the hot plate/EG-bath (caution!) until the liquid in the reaction flask is slightly below the level of the EG. When ethanol begins to boil, start recording the reaction time.
|
2.2.6. Drying of the Biodiesel Samples and Calculation of Biodiesel Yield from Viscosity Measurement
- This part was carried out by the instructors, after the students´ visit. Overnight, the liquid samples in the Falcon tubes usually clearly separate into oil phases and small aqueous phases. For each sample, the upper phase was transferred to a fresh, appropriately marked Falcon tube. These samples were then further dried under reduced pressure at 50°C (Isotemp model 281A, vacuum oven, Fisherbrand) for 6 hours.The BD yields were calculated from the viscosity of the BD samples using a cone-plate rheometer (Brookfield model CAP2000+). The instructors prepared mixtures of the oil and the corresponding BD-100% by weight, ranging from 10 wt% oil/90 wt% BD-100% to 90 wt% oil/10 wt% BD-100%. The instructors measured the viscosities of these 9 samples, plus those of the starting oil and the corresponding BD-100%; all measurements were carried out at 25°C. Plots of these viscosities versus wt% BD-100% in the mixture showed smooth curves, from which the BD yields of the students´ samples were calculated.
2.2.7. Other Activities During the Visits
- During the transesterification reaction, the laboratory technician demonstrated how a gas chromatography equipment works, using Shimadzu GC-2014 FID equipment; the sample injected was an alcoholic solution of oil of clove [14].
3. Results and Discussion
- As given by Equation 3, the students need the values of SV and IV of BD-100% to calculate its CN. These experiments were carried by the students, however, using BO and SO, not the corresponding BD-100%, because this would have required prohibitively large volumes of the pure biodiesel samples. The concepts, however, are the same.
3.1. An Overview of the Activities
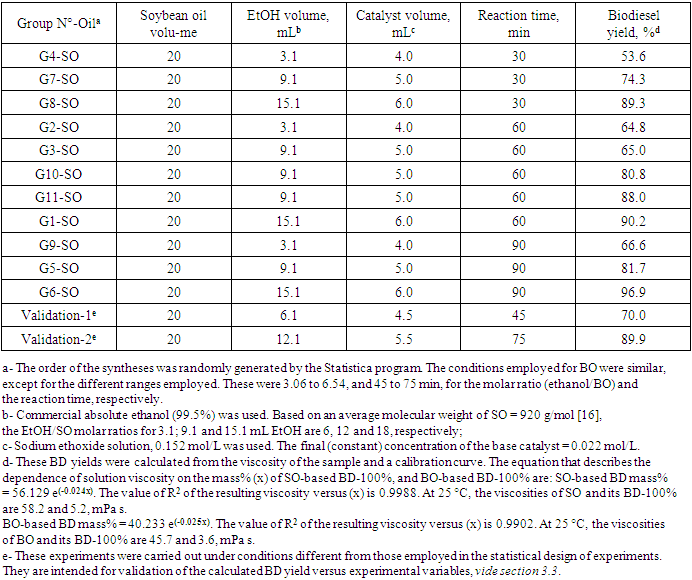
- This project was given to 120 students from five high schools. Its steps include a pre-visit questionnaire to measure the students´ awareness about biofuels in the context of green chemistry; a visit to ChemUSP, discussion of the data obtained in the schools, and a post-visit evaluation. Figure 1 shows details of the activities carried out during the visit; Figure 2 summarizes the students answer to the pre-visit questionnaire; Figure 3 shows the students in the laboratory, whereas Table 1 shows the experimental conditions employed for SO. Addition of the catalyst and start of the reaction
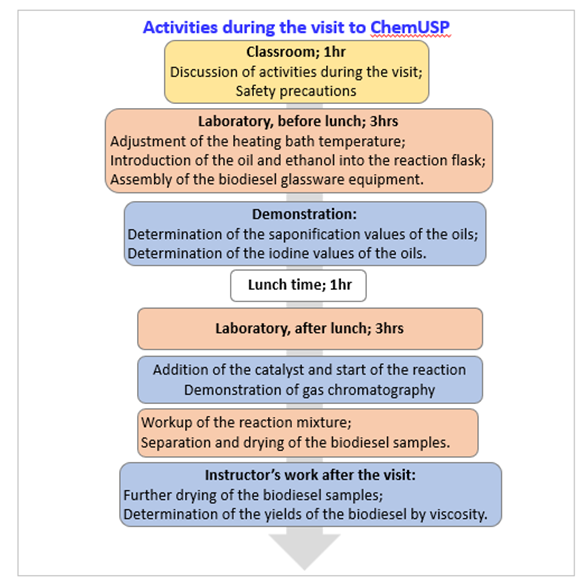 | Figure 1. Activities carried out by high school students during the visit to ChemUSP |
 | Figure 2. Summary of the students’ answer to the pre-visit questionnaire |
 | Figure 3. The students separating the synthesized BD |
3.2. Choice of the Oils and Solving the Emulsion Formation Problem of SO-based BD
- The students noticed major physical differences between the two oils. Thus, BO is transparent and solidifies at room temperature (solidification temperature ca. 24°C, [17]). On the other hand, SO has a golden-yellow color and remains liquid, even on a cold day. The ease of BO solidification can be traced to its highly saturated nature (ca. 89% saturated fatty acids), and is demonstrated by its very small IV (ca. 10 mg iodine/100 g BO; [17]). In Brazil, BO is mainly employed for soap-making, whereas SO is the main vegetable oil for the commercial production of BD [10]. The much higher IV of SO (102 ± 3 g iodine/100 g SO) agrees with those reported elsewhere [18]. This IV was discussed with students in terms of the highly unsaturated nature of this oil, and the reaction employed in the determination of IV, namely, electrophilic addition of iodine to the double bonds of, e.g., esters of oleic and linoleic acids.Crucial to finishing the planned activities is the ready separation of the (BD+SO)/10% NaCl mixture, so that the students can submit their BD samples before the end of their visit. This was the case for BO-based BD, but not for BD from SO. Figure 4 shows the stable water/oil emulsion that formed. Consequently, the students that used SO in the first visit to ChemUSP did not finish their experiment properly!
 | Figure 4. Phase separation of soybean oil-based biodiesel from 10% NaCl solution. The parts on the left and right are for untreated, and pre-treated soybean oil, respectively |
3.3. Use of Statistical Design of Experiments: Determination of the Relative Importance of the Experimental Variables
- As shown by Table 1, the experimental variables investigated were the molar ratio (EtOH/oil) and the reaction time. The concentration of the catalyst and the reaction temperature (the b.p. of EtOH) were kept constant. Two questions arise: What is the effect of each variable on the BD yield? What is the relative importance of each variable? These questions can be conveniently answered by showing the students the plots that we generated by the Statistica software. Figure 5 shows the response surface for the experiments. As shown by the color codes, increasing the values of each variable results in increasing the BD yield.
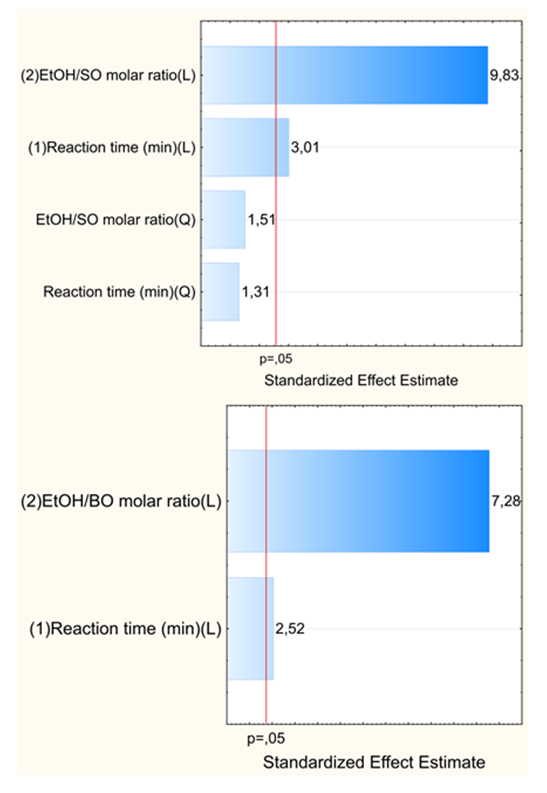 | Figure 6. Pareto plots, showing the dependence of biodiesel yields on the molar ratio (ethanol/oil) and reaction time. The upper plot is for soybean oil, the lower one is for babassu oil |
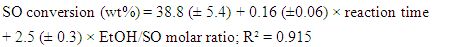 | (4) |
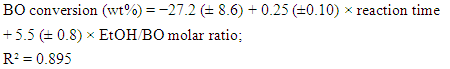 | (5) |
4. Conclusions
- Synthesis of BD from vegetable oils is interesting because it is simple, safe, inexpensive, and is within the scope of ESD; CCE, and green chemistry. The reactions involved, transesterification and electrophilic addition of halogens to double bonds, are given during the chemistry course of the third year of high school students. The use of statistics to optimize a chemical process is a new subject to these students. Depending on the time available, the students can do the whole experimental part. Otherwise, their work should be supplemented by that of the instructors. This (practical) limitation, however, does not affect the education part. Although the formation of stable water/oil emulsions in case of SO was unexpected, it was a “hands-on” experience of how understanding the chemistry behind the problem helps to solve it!
ACKNOWLEDGEMENTS
- We are grateful to the students who participated in this project. We thank Professor Dr. Pedro V. de Oliveira; Professor Dr. Carlos T. Hotta, and members of CCEx of ChemUSP for their support and encouragement; Mr. Alexandre S. Guarezemini (ChemUSP) for his competent technical support in the undergraduate laboratory during all visits, and for demonstrating the gas chromatographic analysis. We thank the administrations of the schools for their support of this activity and Professor Amarílio da Silva Gonçalves from the L. G. Righini high school for his support. We acknowledge FAPESP (grant 2024/13668-4) and CNPq (grants 306108/2019-4; 141853/2019-0, and 131802/2023-2) for research fellowships to O. A. El Seoud, N. Keppeler, and N. R. Vagula, respectively.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML