Todd Wimpfheimer
Department of Chemistry and Physics, Salem State University, Salem MA, USA
Correspondence to: Todd Wimpfheimer, Department of Chemistry and Physics, Salem State University, Salem MA, USA.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Escape rooms have become increasingly popular and have been used at the undergraduate level in a number of fields including chemistry. This paper describes wet chemistry manipulations unique to the chemistry laboratory that can be used in the design of a chemistry laboratory escape room.
Keywords:
Lower division undergraduate, First Year, Escape Room Laboratory, Laboratory experiment
Cite this paper: Todd Wimpfheimer, Twenty Chemistry Tasks in an Hour: Designing a Chemistry Laboratory Escape Room, Journal of Laboratory Chemical Education, Vol. 12 No. 1, 2024, pp. 15-19. doi: 10.5923/j.jlce.20241201.02.
1. Introduction
Escape room experiences are becoming more prevalent in education in general and chemical education in particular. They are fun, student active and can help to introduce or reinforce chemical concepts to students. There are recent examples in the literature of chemistry escape rooms being used in the classroom [1], in general chemistry laboratory [2-6], and in upper level chemistry laboratory courses [7,8]. Chemistry presents the opportunity for students to perform laboratory tasks that would not be possible in a normal room or classroom setting. Most escape room experiences involve guiding students through a set of challenges or puzzles in order to ultimately “escape” the room or else find some ending for the predetermined backstory. In most commercial escape rooms, there are multiple tasks to be done at any one time. Teams are usually given minimal instructions and must work out what is to be done next. Applying these concepts in the chemistry lab was the driving force that started us at Salem State University designing and executing chemistry escape rooms.The purpose of this paper is to aid instructors interested in getting started designing and running chemistry laboratory escape rooms. The chemistry laboratory tasks discussed here are not meant to be an exhaustive list but rather an inventory of easily adopted tasks that can be used to develop a chemistry laboratory escape room appropriate for students at the introductory or general chemistry level.By working together to solve problems under a time constraint and without direct instructions, these escape room activities also help students to practice “soft skills”: time management, collaboration, teamwork, and leadership.
2. Overview
Escape rooms generally present a series of puzzles or tasks that must be solved in order to escape a “locked” room. These puzzles can be deciphering a code to yield a set of numbers or letters which can then be used to unlock a box. Common tasks include putting together a jigsaw puzzle, deciphering a cryptic or hidden message, solving a rebus (in which words are represented by pictures), etc. These types of “dry” challenges can be used in an ordinary classroom, outdoors, and even virtually. However, the current work will focus on chemical laboratory manipulations that can be used in the design of an escape room experience that are unique to the chemistry lab. The “wet” lab manipulations can be combined with “dry” puzzles in order to create a robust escape room experience.At Salem State University, the Chemistry and Physics Department has been using escape room laboratory experiences for about six years in a number of different ways. They have been used as part of the laboratory curriculum, as social events for our chemistry majors, and as recruiting/outreach events for the general university population. (At the depth of the pandemic, virtual chemistry escape rooms were also run for our students to keep them engaged remotely.) These escape room experiences take place in a chemistry laboratory, with the usual laboratory safety precautions. The escape rooms are generally designed for groups of 4-6 people and to last for one hour. Larger groups than this usually result in several passive onlookers. The puzzles, manipulations and challenges are not strictly linear in nature and are designed, like most escape rooms, so that people can be working on different tasks at the same time. Solving the various tasks usually results in a 3 or 4 number or letter code which can be used to open one of the locked boxes/containers in the lab. Some tasks result in obtaining a key which can then be used to open a lock. Also, like commercial escape rooms, students are given access to three “hints” so that they don’t become frustrated. Multiple lockboxes and locks are required. Some of the fun of escape rooms is encountering different types of locks that must be opened, so the design should utilize many kinds of locks such as numerical, alphabetical, directional, and key. The instructor(s) are observing the students, circulating around the room and overseeing some of the tasks as necessary. Most of the students have been generally familiar with basic laboratory safety and appropriate reading of basic lab equipment. If however, a student group have never been in a chemistry lab before, they will require preliminary instruction before the tasks. What follows is a description of some of the wet laboratory tasks that have been used at Salem State in the design of chemistry laboratory escape room experiences. The manipulations are designed to be at the general chemistry level. For any one escape room experience roughly 10-12 of these wet tasks are mixed with 8-10 dry tasks to produce a fun, diverse and valuable chemistry experience.
3. Lab Tasks
The following is a list of some of the more common general chemistry lab tasks that have been used in our escape room experiences. The tasks may be mixed and matched depending on the chemicals and equipment that are available. Most are fairly easy to set up. LAB SAFETY AND CLEALINESS – These tasks help to enforce lab safety and cleanliness guidelines to the students.Clean up a Spill – A 4 digit number code is taped to the lab bench and then covered with spilled solid. The solid used can by as simple as NaCl or it can be a more complicated (and prettier) chemical like Cu(NO3)2. Nearby is a list of safety rules with the phrase “all spills must be cleaned up” highlighted. Students can be told what the substance is and/or what the proper disposal should be.  | Figure 1. Spill task |
Dirty Equipment – The same or another list of safety rules can have the phrase “all glassware and equipment must be kept clean” highlighted. Nearby can be a crucible or flask with a 4 digit number code written on the inside bottom and then again covered with a solid. This helps introduce students to common laboratory equipment like crucibles, beakers or Erlenmeyer flasks. A code taped to beaker tongs and then dirtied up has also been used.Broken Glassware – The same or another list of safety rules can have the phrase “broken glass must be placed in the broken glass container”. When the glass is swept up, a code is revealed. This also introduces the student to the location of dustpan, broom, and broken glass container.Eyewash/Safety Shower Key – A message either revealed when a box is unlocked or in plain sight can read “Make sure you know the location of the safety eyewash”. When the students inspect the eyewash, they should discover a lightly hidden key taped there which opens another lock. The safety shower has also been used to hide a key.Fume Hood Key – A message either revealed when a box is unlocked or in plain sight can read “Make sure you know the location of the fume hood”. When the students inspect the hood, they should discover a lightly hidden key taped there.LAB MEASUREMENTSThese tasks help to introduce students to some basic laboratory equipment and measurements.Equipment – A message either revealed when a box is unlocked or in plain sight can read: “beaker, Erlenmeyer flask, watch glass, test tube in that order”. Nearby or in the unlocked box are those pieces of equipment with each one being labelled 1, 2, 3, or 4. Putting them in the correct order reveals a code that can open another lock. This is used to familiarize students with common laboratory equipment. Any other pieces of equipment like a buret stand, thermometer clamp, stirrer/hotplate, etc. can be substituted.Graduated Cylinder/Buret - A message either revealed when a box is unlocked or in plain sight can read “Measuring the volume of the 10-mL graduated cylinder to the correct number of significant figures will reveal the code:__ . __ __ “ Nearby there is a parafilmed 10-mL graduated cylinder containing a certain amount of water. This introduces the student to both common lab glassware and the concept of significant figures. A 50-mL buret has also been used for a 4 digit code.Significant Figures of Various Glassware – A 50-mL buret, a 10-mL graduated cylinder, and a 100-mL graduated cylinder are filled with water to various levels and placed in order. A message either revealed when a box is unlocked or in plain sight can read “The number of significant figures used for each of these four measuring devices in order will yield the code: __ __ __ _”.Again, this can be used to introduce students to various pieces of glassware and their differing levels of precision.Measuring Mass – An unlocked box can reveal a small cube of metal with the message “Measuring the mass on the top loading balance will reveal the code: __ __. __ __” . This introduces the student to the operation of the balance. The balance is not zeroed beforehand to emphasize the concept of zeroing. The analytical balance has also been used.Measuring Density – An unlocked box can reveal a small cylinder of metal (we use aluminum) with the message “the measurement of the density of this metal in g/mLwill reveal the code __ . __ __ “. A graduated cylinder is usually placed nearby. This exposes the student to density measurement, units of density and the concept of displaced volume. A small cube whose volume can be determined by measuring length, width, and height has also been used.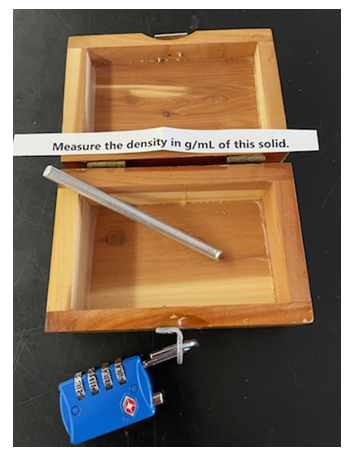 | Figure 2. Density task |
COMBUSTION - Students like to set things on fire, so we always include a way for them to do so safely. The instructor should be on hand supervising these combustion tasks.Burning Salts– A message either revealed when a box is unlocked or in plain sight can read “Go to the Bunsen burner and burn the four salts there; the sample numbers in order of increasing wavelength will yield the code: __ __ __ __”. Nearby is a Bunsen burner set up with 4 vials containing solid samples. We use KCl, NaCl, Sr(NO3)2, and Cu(NO3)2 labelled 1 ,2, 3, and 4. This is a visually stunning display and is usually one of the students’ favorite tasks. It introduces them to the operation of a Bunsen burner and also the varying wavelengths of colored light. Alcohol solutions of these salts have also been used.Burning Mg - A message either revealed when a box is unlocked or in plain sight can read “Do not use force! Some locks are ignitable”. In plain sight on a nearby lab bench is a small metal box that is fastened closed with a strip of magnesium ribbon. There is also a nearby butane lighter. The students can be reminded not to stare directly at the magnesium as it is burning. Alternatively, we have used 3 strips of Mg in a sand bath as hints in case the students get stuck. If they want a hint, all they have to do is light the Mg strip.  | Figure 3. Burning Mg |
ACIDS AND BASES – These tasks involve elementary acid/base reactions and pH.pH Paper – A box is unlocked and contains 3 liquid sample vials labeled 1, 2, and 3. One contains HCl(aq), one contains NaOH(aq), and one contains water. There is also the clue: “Use universal pH paper to help crack the code:neutral, acid, base __ __ __” . This reminds the student of the pH scale and acidic, basic, and neutral solutions. pH Secret Message– A box is unlocked and contains a small Erlenmeyer flask containing NaOH(aq) made dark pink by the addition of phenolphthalein, along with the clue: “Lower the pH of the pink solution until the secret message is revealed”. On the bottom of the flask written in pink permanent marker is a secret message. It can be a location in lab where a key is hidden or a 4 digit code that will unlock another box. There are nearby bottles of water, HCl(aq), NaOH(aq). Addition of acid decolorizes the solution and allows the message to be seen. Acid/Base Volcano - A box is unlocked and contains a small labelled vial of vinegar and the message: “Add vinegar all at once to the volcano and a secret code will be revealed”. Nearby is a “volcano”, an inverted plastic funnel covering a 10-mL beaker half-filled with baking soda. Once the vinegar is added, the volcano “erupts” and spits out a laminated code. On one occasion the code landed perfectly on top of the volcano as shown in Figure 4. The students usually enjoy this task very much. Pouring diet coke into the volcano which contains mentos candies has also been used. | Figure 4. Acid/Base Volcano |
OTHER TASKSLiquid Nitrogen – A box is unlocked revealing an inflated balloon, and the message: “Do not touch the balloon. Shrink this balloon to reveal a secret message. You’ll need something very cold…about 77 K “. There is a nearby Dewar flask filled with liquid nitrogen and cryogenic gloves. When liquid nitrogen is poured on the balloon, it shrinks, revealing a message or code written at the bottom of the container. Most general chemistry students have not handled liquid nitrogen before so they will require oversight performing this task.Melting Gallium – A box is unlocked containing a solid sample of gallium in a Petri dish that has a laminated code within it. The box also contains the message: “A secret code is contained within this sample of gallium, melting point of 29.8°C.” A butane lighter, Bunsen burner and ring stand are suggestively placed nearby. When the sample is melted, the code can be extracted. This task can be run in the hood if desired. Students enjoy seeing this metal melt so easily.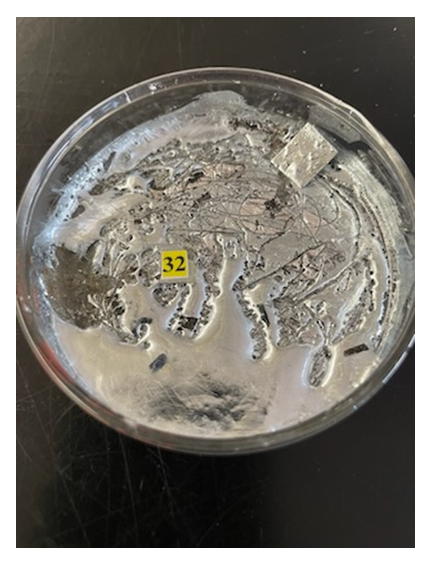 | Figure 5. Melting gallium |
Mel-Temp – A box is unlocked containing 4 sample vials labeled, “first digit”, “second digit”, “third digit”, and “fourth digit”. The samples are lauric acid (mp=43°C), palmitic acid (mp=64°C), aspirin (mp=135°C), and water. There is also a message and a picture of a thermometer with different temperature ranges numbered 1,2,3,4,5. “Use a Mel-Temp to determine the melting point of each of these samples to yield this four digit code” __ __ __ __.Students must figure out that liquid water has already “melted”. Students gain experience using the Mel-Temp apparatus.Spec-200 – A box is unlocked containing a labelled sample of KMnO4(aq), and the message: “Use a Spec-200 UV-Vis spectrophotometer to determine the wavelength in nm that gives the maximum absorbance of this sample to determine the code: __ __ __.” Nearby there is a Spec-200 UV-Vis spectrophotometer and cuvettes. The student gains experience using a UV-Vis spectrophotometer.Styrofoam Key – A box is unlocked containing a small covered jar holding a key lodged in Styrofoam. It has a lid with a small hole in it. There is also the message: “Do not open jar! Use acetone to dissolve the Styrofoam”. Nearby there is a bottle of acetone and a magnetic stir bar retriever. Once acetone is used to dissolve the Styrofoam, the key can be retrieved with the magnet. Additionally, water soluble packing peanuts and water have also been used.
4. Hazards
HCl(aq) and NaOH(aq) are corrosive. Cu(NO3)2(s) and KCl(s) are irritants and should not be ingested. KMnO4(aq) is an oxidizing agent. Acetone and magnesium metal are flammable. Liquid nitrogen can cause frostbite or severe cryogenic burns. Sr(NO3)2(s) is an oxidizer and irritant and should not be ingested. Lauric acid and palmitic acid may cause irritation to skin and eyes. Gallium should not be ingested. Students should wear protective eyewear at all times during the laboratory and gloves when necessary
5. Discussion
Chemical laboratory escape rooms using these and other puzzles have been developed and run at Salem State for several years now. One particular escape room has become a core experiment in Physical Chemistry I [9]. Escape rooms are run periodically throughout the academic year as recruiting/outreach events as well as events for our chemistry majors. These events are well received by the students who participate in them. One thing that is important for students participating in an escape room event is the prize at the end of it. Sometimes just doing it in the fastest time is prize enough. The prizes that we have used for various events have included safety glasses, American Chemical Society chemical element pins [10], 50-mL plastic beakers and small denomination gift cards. During the campus dedensification owing to the pandemic, when most lectures were delivered via Zoom, we offered computer camera covers as prizes. But by far, the most popular prize with the students is extra credit points on a quiz or homework.Escape room chemical laboratory events are fun and a unique way to introduce or reinforce laboratory techniques. Students get to practice handling glassware, disposing of chemical waste, making measurements, and performing chemical manipulations. Students also get to work on several soft skills as well such as collaboration, communication, and time management.
ACKNOWLEDGEMENTS
The author thanks Leah Wimpfheimer for helpful discussions.
References
| [1] | Doughty, R, 2024, Project: Lockbox- A reusable escape-room-style activity for the classroom, J. Chem. Educ. (101) 2, 682-686. |
| [2] | Peleg, R., Yayon, M., Katchevich D., Moria-Shipony, M., and Blonder, R., 2019, A lab-based chemical escape room: educational, mobile, and fun! J. Chem. Educ., (96) 955-960. |
| [3] | Watermeier, D., and Salzameda, B., 2019, Escaping boredom in first semester general chemistry, J. Chem. Educ., (96) 961-964. |
| [4] | Nguyen, T., 2018, Chemistry education moves from classroom to escape room, Chem. Eng. News, (32) 28-30. |
| [5] | Avargil, S., Shwartz, G., and Zemel, Y., 2021, Educational Escape Room: Break Dalton’s code and escape!, J. Chem. Educ., (98) 7, 2313-2322. |
| [6] | Mahomed, M., Sisodia, L., Williams, D., and Villa-Marcos, B., 2024, Spectroscopy unlocked: an escape room activity for introductory chemistry courses, J. Chem. Educ., (101) 6, 2570-2575. |
| [7] | Musgrove, H., Ward, W., and Hiatt, L., 2021, Escape from quant. Lab: using lab skill progression and a final project to engage students, J. Chem. Educ. (98) 7, 2307-2312. |
| [8] | Vergne, M., Simmons, J., and Bowen, R., 2019, Escape the lab: an interactive escape room game as lab experiment, J. Chem. Educ., (96) 985-991. |
| [9] | Wimpfheimer, T., 2020, A physical chemistry escape room laboratory: is Schrodinger’s cat dead and/or alive?, J Laboratory Chemical Education 8(1) 1-5. |
| [10] | American Chemical Society store https://www.store.acs.org/eweb/ACSTemplatePage.aspx? site=ACS_Store&WebCode=storeCatList&catKey=ee5070ef-6c3a-4f02-959a-12a84fff4ade (accessed July 15, 2024). |







 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML