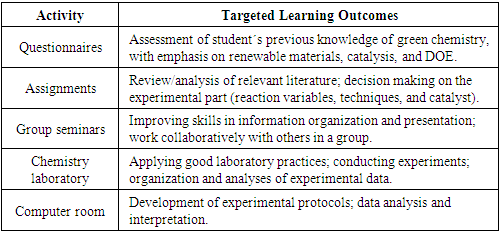
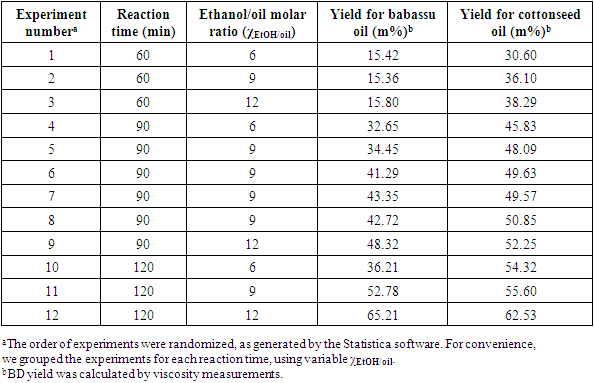
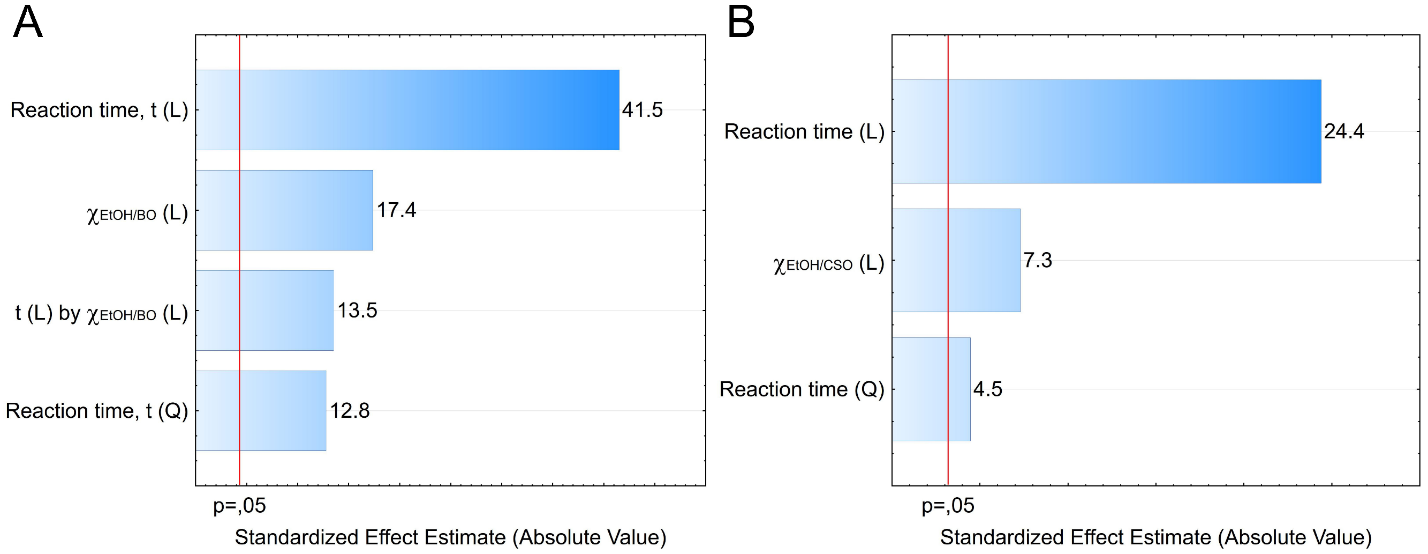
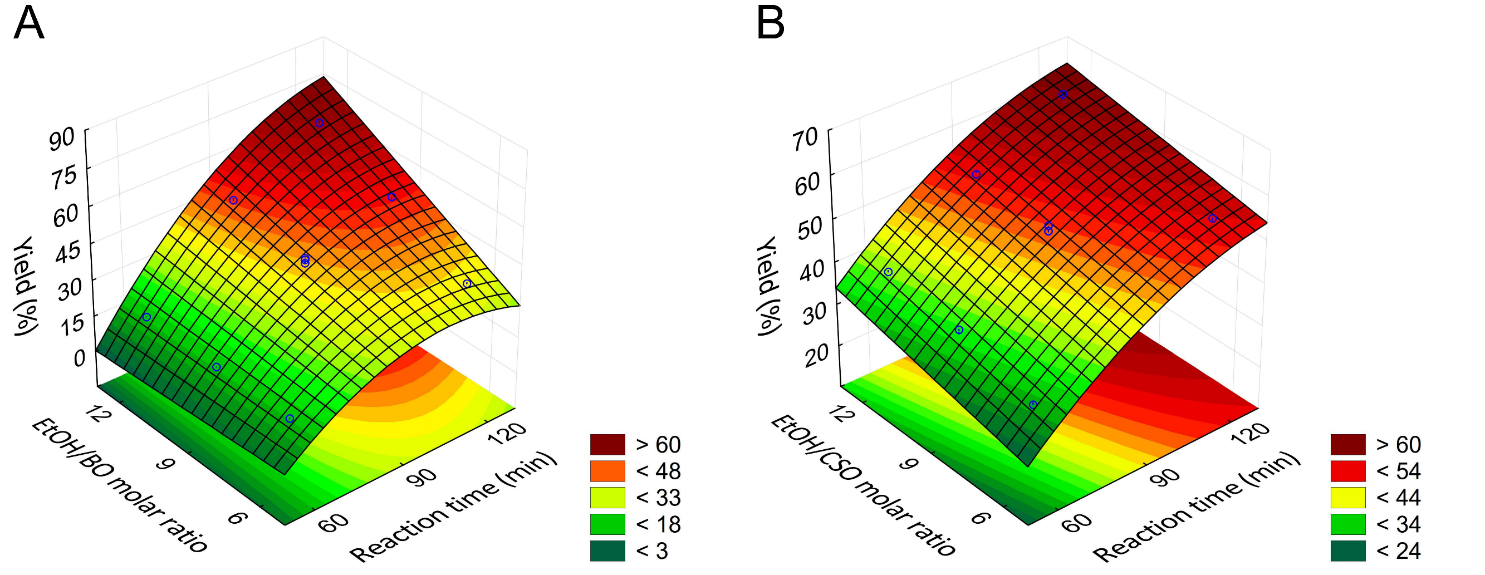
| [1] | The Future of Diesel: Is Diesel Going Away? Detroit Diesel Corporation. (2023). https://www.demanddetroit.com/our-company/community/blog/the-future-of-diesel-is-diesel-going-away. (accessed January 12, 2024). |
| [2] | Diesel Is Not Dead; It’s Part of the Path Forward, Cummins Newsrooom. (2022). https://www.cummins.com/news/2022/08/16/diesel-not-dead-its-part-path-forward. (accessed January 12, 2024). |
| [3] | Economics and Industry Data, American Trucking Associations. (2022). https://www.trucking.org/economics-and-industry-data. (accessed January 12, 2024). |
| [4] | Bureau of Infrastructure and Transport Research Economics Statistical Report, Australian Infrastructure and Transport Statistics Yearbook 2022. (2022). https://www.bitre.gov.au/sites/default/files/documents/bitre-yearbook-2022.pdf. (accessed January 12, 2024). |
| [5] | Confederação Nacional do Transporte, Anuário CNT do Transporte. (2022). https://anuariodotransporte.cnt.org.br/2022/Rodoviario/1-1-/Inicial. (accessed January 12, 2024). |
| [6] | Xu, H.; Ou, L.; Li, Y.; Hawkins, T. R.; Wang, M. Life Cycle Greenhouse Gas Emissions of Biodiesel and Renewable Diesel Production in the United States. Environ Sci Technol 2022, 56 (12), 7512–7521. https://doi.org/10.1021/acs.est.2c00289. |
| [7] | Samuel, O. D.; Oreko, B. U.; Oyejide, J. O.; Idi, S.; Fayomi, O. S. I. Experimental and Empirical Study of Diesel and Biodiesel Produced from Blend of Fresh Vegetable and Waste Vegetable Oil on Density, Viscosity, Sulphur Content and Acid Value. J Phys Conf Ser 2019, 1378 (4), 042024. https://doi.org/10.1088/1742-6596/1378/4/042024. |
| [8] | Traviss, N.; Thelen, B. A.; Ingalls, J. K.; Treadwell, M. D. Biodiesel versus Diesel: A Pilot Study Comparing Exhaust Exposures for Employees at a Rural Municipal Facility. J Air Waste Manage Assoc 2010, 60 (9), 1026–1033. https://doi.org/10.3155/1047-3289.60.9.1026. |
| [9] | Vendas de Combustíveis por Distribuidoras Crescem 2,5% em 2022; Diesel Bate Recorde, Diz ANP, CNN Brasil. (2023). https://www.cnnbrasil.com.br/economia/vendas-de-combustiveis-por-distribuidoras-crescem-25-em-2022-diesel-bate-recorde-diz-anp/. (accessed January 12, 2024). |
| [10] | UNESCO Roadmap for Implementing the Global Action Program on Education for Sustainable Development, UNESCO. (2014). https://unesdoc.unesco.org/ark:/48223/pf0000230514. |
| [11] | Education is Key to Addressing Climate Change, United Nations. https://www.un.org/en/climatechange/climate-solutions/education-key-addressing-climate-change. (accessed January 12, 2024). |
| [12] | Facts About the Climate Emergency https://www.unep.org/facts-about-climate-emergency. (accessed January 12, 2024). |
| [13] | Serra, J. L.; Rodrigues, A. M. da C.; de Freitas, R. A.; Meirelles, A. J. de A.; Darnet, S. H.; Silva, L. H. M. da. Alternative Sources of Oils and Fats from Amazonian Plants: Fatty Acids, Methyl Tocols, Total Carotenoids and Chemical Composition. Food Research International 2019, 116, 12–19. https://doi.org/10.1016/j.foodres.2018.12.028. |
| [14] | Riaz, T.; Iqbal, M. W.; Mahmood, S.; Yasmin, I.; Leghari, A. A.; Rehman, A.; Mushtaq, A.; Ali, K.; Azam, M.; Bilal, M. Cottonseed Oil: A Review of Extraction Techniques, Physicochemical, Functional, and Nutritional Properties. Crit Rev Food Sci Nutr 2023, 63 (9), 1219–1237. https://doi.org/10.1080/10408398.2021.1963206. |
| [15] | Grote, M., & Schildmann-Humberg, P. (1995). Micro determinations of capacity values by pH titration: a critical analytical investigation. Industrial & Engineering Chemistry Research, 34(8), 2712–2718. https://doi.org/10.1021/ie00047a021. |
| [16] | Pinheiro, A.; Gonçalves, A.; Baader, W.; Yamaguchi, L.; Malek, N.; Bastos, E.; Seoud, O. Biofuels From Coconut Fat And Soybean Oil: Microwave-Assisted Synthesis and Gas Chromatography/Mass Spectrometry ANALYSIS. Quim Nova 2018. https://doi.org/10.21577/0100-4042.20170273. |
| [17] | Shibasaki-Kitakawa, N.; Honda, H.; Kuribayashi, H.; Toda, T.; Fukumura, T.; Yonemoto, T. Biodiesel Production Using Anionic Ion-Exchange Resin as Heterogeneous Catalyst. Bioresour Technol 2007, 98 (2), 416–421. https://doi.org/10.1016/j.biortech.2005.12.010. |
| [18] | El Seoud, O. A.; Keppeler, N.; Sandrini, D. M. F.; Morselli, G. R. Active Learning in the Undergraduate Chemistry Laboratory: Synthesis of Biodiesel from an Amazon Region Oil (Babassu): Keep It Simple! J Chem Educ 2023, 100 (8), 2926–2934. https://doi.org/10.1021/acs.jchemed.2c01124. |
| [19] | Food Safety and Standard Authority of India. Manual of Methods of Analysis of Foods: Oils and Fats. Ministry of Health and Family Welfare. FSSAI: New Delhi 2015, pp 1–86. https://www.fssai.gov.in/upload/uploadfiles/files/OILS_AND_FAT.pdf. (accessed January 12, 2024). |
| [20] | Tubino, M.; Aricetti, J. A. A Green Potentiometric Method for the Determination of the Iodine Number of Biodiesel. Fuel 2013, 103, 1158–1163. https://doi.org/10.1016/j.fuel.2012.10.011. |
| [21] | MONTEIRO, M.; AMBROZIN, A.; LIAO, L.; FERREIRA, A. Critical Review on Analytical Methods for Biodiesel Characterization. Talanta 2008, 77 (2), 593–605. https://doi.org/10.1016/j.talanta.2008.07.001. |
| [22] | Nasir, N. F.; Daud, W. R. W.; Kamarudin, S. K.; Yaakob, Z. Methyl Esters Selectivity of Transesterification Reaction with Homogenous Alkaline Catalyst to Produce Biodiesel in Batch, Plug Flow, and Continuous Stirred Tank Reactors. International Journal of Chemical Engineering 2014, 2014, 1–13. https://doi.org/10.1155/2014/931264. |
| [23] | Pérez-Méndez, M. A.; Jiménez-García, G.; Huirache-Acuña, R.; Maya-Yescas, R. Estimation of Reaction Rates of Transesterification Pathways. Frontiers in Chemical Engineering 2021, 3. https://doi.org/10.3389/fceng.2021.673970. |
| [24] | Teixeira, M. A. Heat and Power Demands in Babassu Palm Oil Extraction Industry in Brazil. Energy Convers Manag 2005, 46 (13–14), 2068–2074. https://doi.org/10.1016/j.enconman.2004.10.014. |
| [25] | Isaac, I. O.; Ekpa, O. D. Fatty Acid Composition of Cottonseed Oil and Its Application in Production and Evaluation of Biopolymers. American Journal of Polymer Science 2013, 2013 (2), 13–22. https://doi.org/10.5923/j.ajps.20130302.02. |
| [26] | Silva, M. J. F. da; Rodrigues, A. M.; Vieira, I. R. S.; Neves, G. de A.; Menezes, R. R.; Gonçalves, E. da G. do R.; Pires, M. C. C. Development and Characterization of a Babassu Nut Oil-Based Moisturizing Cosmetic Emulsion with a High Sun Protection Factor. RSC Adv 2020, 10 (44), 26268–26276. https://doi.org/10.1039/D0RA00647E. |
| [27] | Zia, M. A.; Shah, S. H.; Shoukat, S.; Hussain, Z.; Khan, S. U.; Shafqat, N. Physicochemical Features, Functional Characteristics, and Health Benefits of Cottonseed Oil: A Review. Brazilian Journal of Biology 2022, 82. https://doi.org/10.1590/1519-6984.243511. |
| [28] | Onukwuli, D. O.; Emembolu, L. N.; Ude, C. N.; Aliozo, S. O.; Menkiti, M. C. Optimization of Biodiesel Production from Refined Cotton Seed Oil and Its Characterization. Egyptian Journal of Petroleum 2017, 26 (1), 103–110. https://doi.org/10.1016/j.ejpe.2016.02.001. |
| [29] | What is a Good Cetane Number – and Why is it Important? FleetOwner. (2021). https://www.fleetowner.com/shifting-gears/article/21161693/what-is-a-good-cetane-number-and-why-is-it-important. (accessed January 12, 2024). |
| [30] | Halvorsen, J.D., W.C. Mammel Jr., and L.D. Clements, Density Estimation of Fatty Acids and Vegetable Oils Based on Their Fatty Acid Composition, J. Am. Oil Chem. Soc. 70: 875–880 (1993). https://doi.org/10.1007/bf02545346. |
| [31] | Rodenbush, C. Hsieh, F. Viswanath D. Density and viscosity of vegetable oils, Journal of the American Oil Chemists' Society, (1999), 1415-1419, 76(12) (1999). https://doi.org/10.1007/s11746-999-0177-1. |
| [32] | Folayan, A. Anawe, P. Aladejare, Ayeni, A. Experimental investigation of the effect of fatty acids configuration, chain length, branching and degree of unsaturation on biodiesel fuel properties obtained from lauric oils, high-oleic and high-linoleic vegetable oil biomass, Energy Reports, (2019), 793-806, 5. https://doi.org/10.1016/j.egyr.2019.06.013. |
| [33] | Fasina, O. O.; Hallman, H.; Craig-Schmidt, M.; Clements, C. Predicting Temperature-dependence Viscosity of Vegetable Oils from Fatty Acid Composition. J Am Oil Chem Soc 2006, 83 (10), 899–903. https://doi.org/10.1007/s11746-006-5044-8. |
| [34] | Cho, Y.-J.; Kim, T.-E.; Gil, B. Correlation between Refractive Index of Vegetable Oils Measured with Surface Plasmon Resonance and Acid Values Determined with the AOCS Official Method. LWT - Food Science and Technology 2013, 53 (2), 517–521. https://doi.org/10.1016/j.lwt.2013.03.016. |
| [35] | Zhao, L.; Riensche, E.; Blum, L.; Stolten, D. Multi-Stage Gas Separation Membrane Processes Used in Post-Combustion Capture: Energetic and Economic Analyses. J Memb Sci 2010, 359 (1–2), 160–172. https://doi.org/10.1016/j.memsci.2010.02.003. |
| [36] | Atadashi, I. M.; Aroua, M. K.; Aziz, A. A. Biodiesel Separation and Purification: A Review. Renew Energy 2011, 36 (2), 437–443. https://doi.org/10.1016/j.renene.2010.07.019. |
| [37] | Suthar, K.; Dwivedi, A.; Joshipura, M. A Review on Separation and Purification Techniques for Biodiesel Production with Special Emphasis on Jatropha Oil as a Feedstock. Asia-Pacific Journal of Chemical Engineering 2019, 14 (5). https://doi.org/10.1002/apj.2361. |
| [38] | Pareto Principle (80/20 Rule) & Pareto Analysis Guide, Juran. (2019). https://www.juran.com/blog/a-guide-to-the-pareto-principle-80-20-rule-pareto-analysis/. (accessed January 12, 2024). |
| [39] | Falco, M. G.; Córdoba, C. D.; Capeletti, M. R.; Sedran, U. Basic Ion Exchange Resins as Heterogeneous Catalysts for Biodiesel Synthesis. Adv Mat Res 2010, 132, 220–227. https://doi.org/10.4028/www.scientific.net/AMR.132.220. |
| [40] | Doudin, K. I. Quantitative and Qualitative Analysis of Biodiesel by NMR Spectroscopic Methods. Fuel 2021, 284, 119114. https://doi.org/10.1016/j.fuel.2020.119114. |
| [41] | Vlahov, G. Quantitative Method Using the 13C NMR DEPT Pulse Sequence for the Detection of Olive Oil Adulteration with Soybean Oil. Magnetic Resonance in Chemistry, 1997, 35, 8–12. https://doi.org/10.1002/(SICI)1097-458X(199712)35:13. |
| [42] | Mohd Azhar, S. H.; Abdulla, R.; Jambo, S. A.; Marbawi, H.; Gansau, J. A.; Mohd Faik, A. A.; Rodrigues, K. F. Yeasts in Sustainable Bioethanol Production: A Review. Biochem Biophys Rep 2017, 10, 52–61. https://doi.org/10.1016/j.bbrep.2017.03.003. |



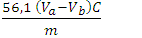
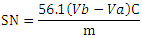
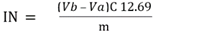
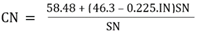
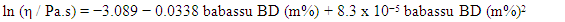
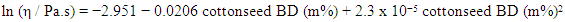
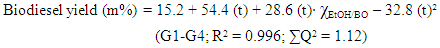
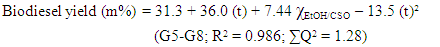
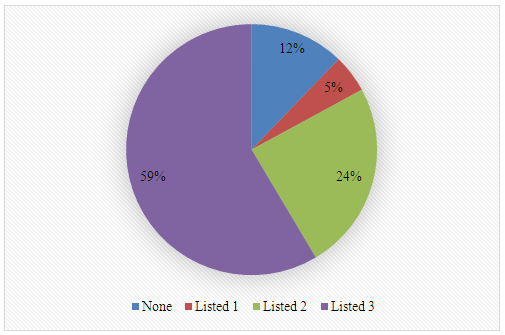 Oils listed by students: Avocado, cotton, almond, peanut, rice, babassu, buriti, cocoa, canola, chestnut, Brazil nut, coconut, copaiba, cupuaçu, shea, flaxseed, castor, melon, murumuru, olive, palm, peroba, primrose, and castor oil.2. What is your previous contact with biodiesel?
Oils listed by students: Avocado, cotton, almond, peanut, rice, babassu, buriti, cocoa, canola, chestnut, Brazil nut, coconut, copaiba, cupuaçu, shea, flaxseed, castor, melon, murumuru, olive, palm, peroba, primrose, and castor oil.2. What is your previous contact with biodiesel?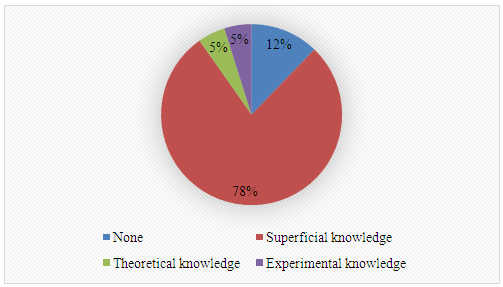 3. Name two instrumental techniques, either single or tandem, that can be used in the analysis of biodiesel.
3. Name two instrumental techniques, either single or tandem, that can be used in the analysis of biodiesel.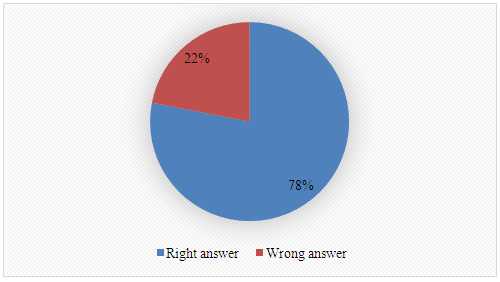 Techniques listed by students: Gas Chromatography (GC), High-Performance Liquid Chromatography (HPLC), Nuclear Magnetic Resonance (NMR), UV-Visible Spectroscopy (UV-Vis), Rheometry, Infrared Spectroscopy, Gravimetry, Gas Chromatography-Mass Spectrometry (GC-MS), High-Performance Liquid Chromatography-Mass Spectrometry (HPLC-MS).4. What is your previous contact with catalysis of transesterification reactions?
Techniques listed by students: Gas Chromatography (GC), High-Performance Liquid Chromatography (HPLC), Nuclear Magnetic Resonance (NMR), UV-Visible Spectroscopy (UV-Vis), Rheometry, Infrared Spectroscopy, Gravimetry, Gas Chromatography-Mass Spectrometry (GC-MS), High-Performance Liquid Chromatography-Mass Spectrometry (HPLC-MS).4. What is your previous contact with catalysis of transesterification reactions?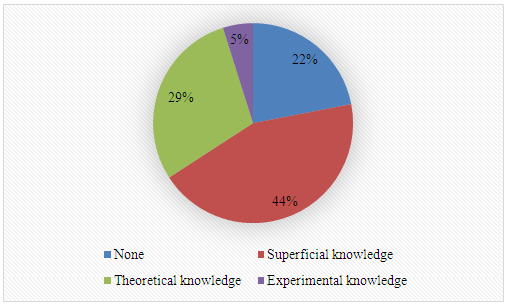 5. What is your previous contact with chemometrics?
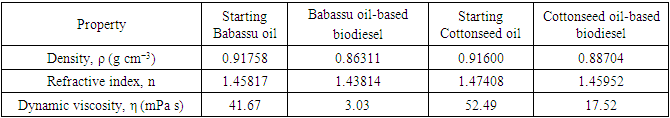
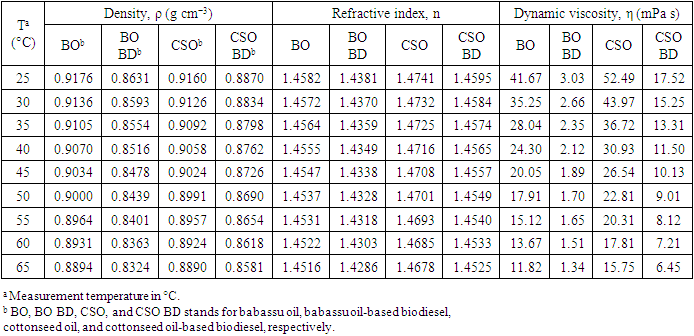
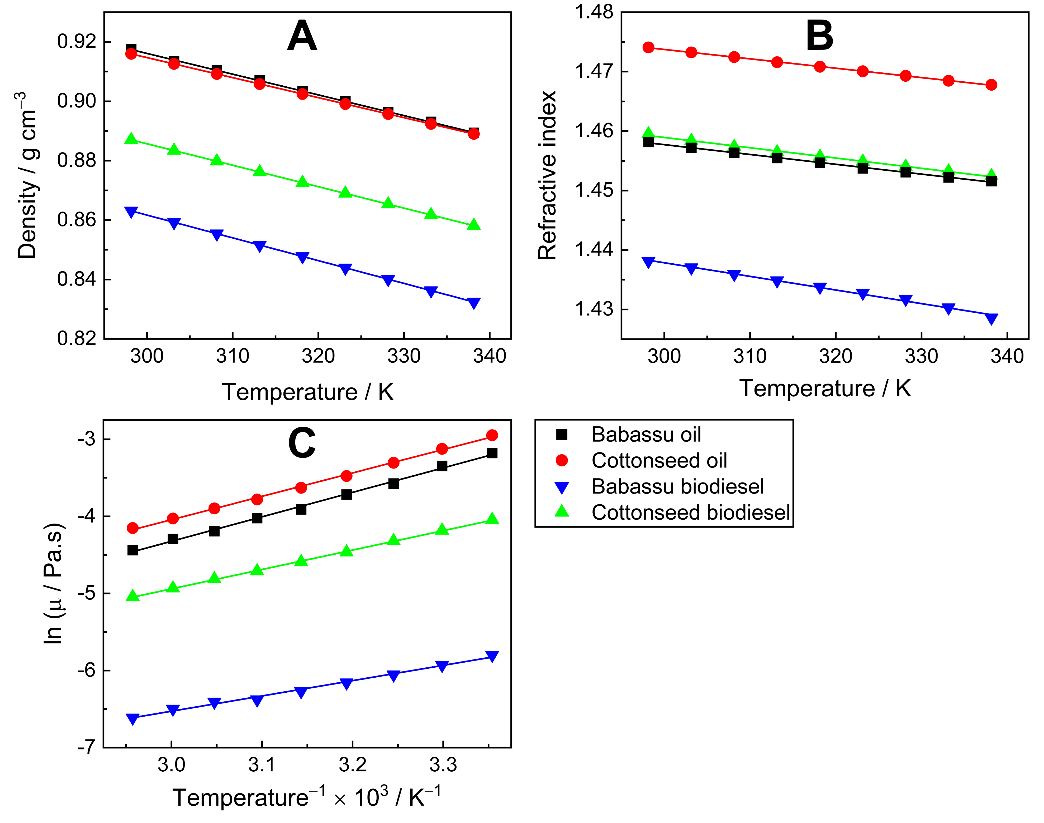
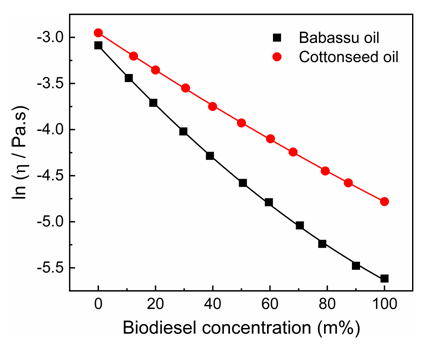
5. What is your previous contact with chemometrics?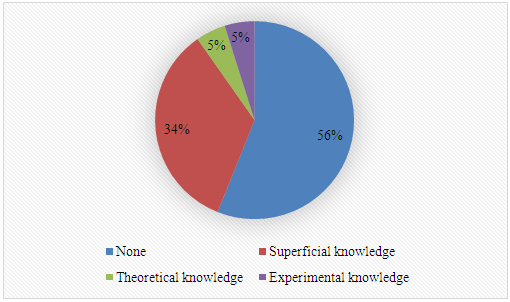 SI-2-Questionnaire 2Questionnaire 2: Decision on Alternative Techniques for Biodiesel Yield DeterminationAt the end of the transesterification reaction of a vegetable oil, the reaction mixture was repeatedly washed with water to remove excess ethanol, catalyst, and the glycerol produced. This leaves product containing unreacted vegetable oil and the corresponding biodiesel. The next step is to determine the composition of this product to determine the yield of biodiesel.Several techniques can be used, e.g. gas chromatography alone or coupled with a mass detector are robust techniques to determine the biodiesel yield. However, our interest is to use another, simple instrument.So far, you have determined the density (ρ), refractive index (n), and dynamic viscosity (η) of babassu and cottonseed oil. The following Table shows the values of these properties for babassu oil, cottonseed oil, and the corresponding (authentic) biodiesels.
SI-2-Questionnaire 2Questionnaire 2: Decision on Alternative Techniques for Biodiesel Yield DeterminationAt the end of the transesterification reaction of a vegetable oil, the reaction mixture was repeatedly washed with water to remove excess ethanol, catalyst, and the glycerol produced. This leaves product containing unreacted vegetable oil and the corresponding biodiesel. The next step is to determine the composition of this product to determine the yield of biodiesel.Several techniques can be used, e.g. gas chromatography alone or coupled with a mass detector are robust techniques to determine the biodiesel yield. However, our interest is to use another, simple instrument.So far, you have determined the density (ρ), refractive index (n), and dynamic viscosity (η) of babassu and cottonseed oil. The following Table shows the values of these properties for babassu oil, cottonseed oil, and the corresponding (authentic) biodiesels.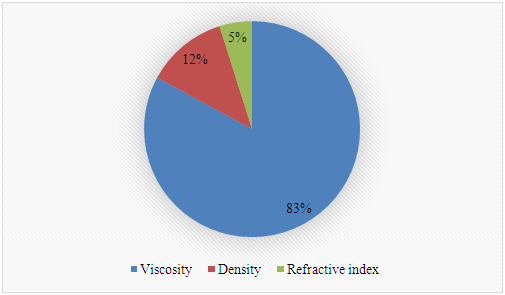 Q-2.2 - Explain how you would carry out the experiment to determine the yield of biodiesel. Break your answer into steps: 1, 2, 3, etc.
Q-2.2 - Explain how you would carry out the experiment to determine the yield of biodiesel. Break your answer into steps: 1, 2, 3, etc. Q-2.3 - Given your choice of technique, briefly discuss an advantage and a potential practical limitation of using it to determine the yield of biodiesel.
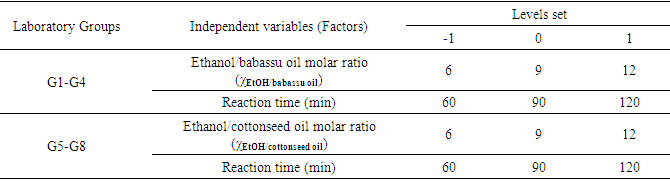
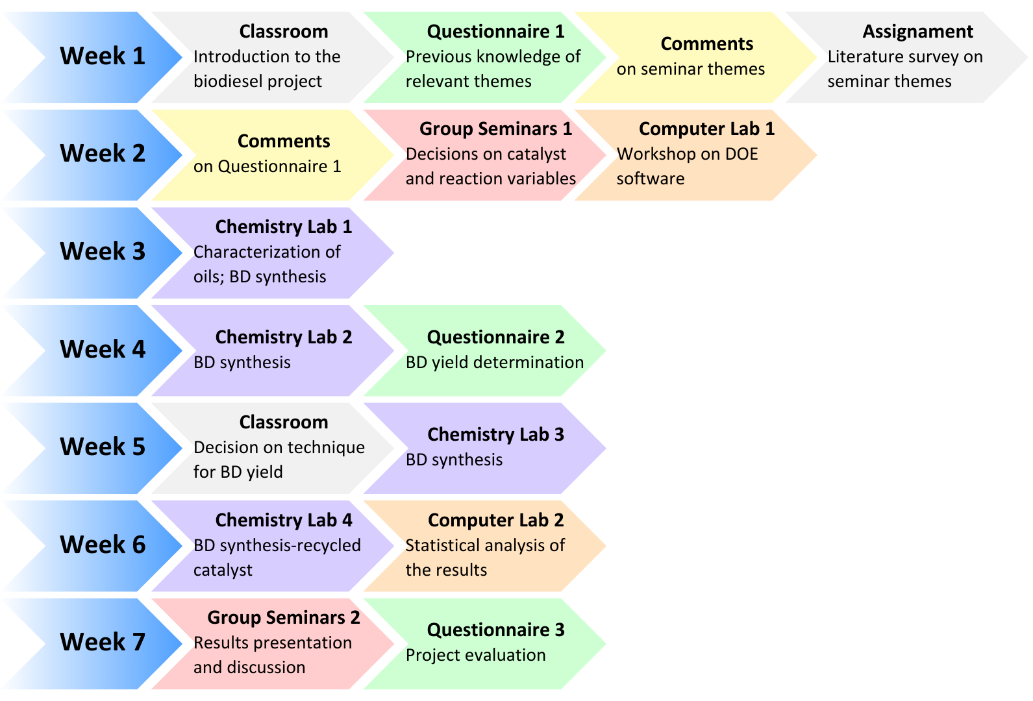
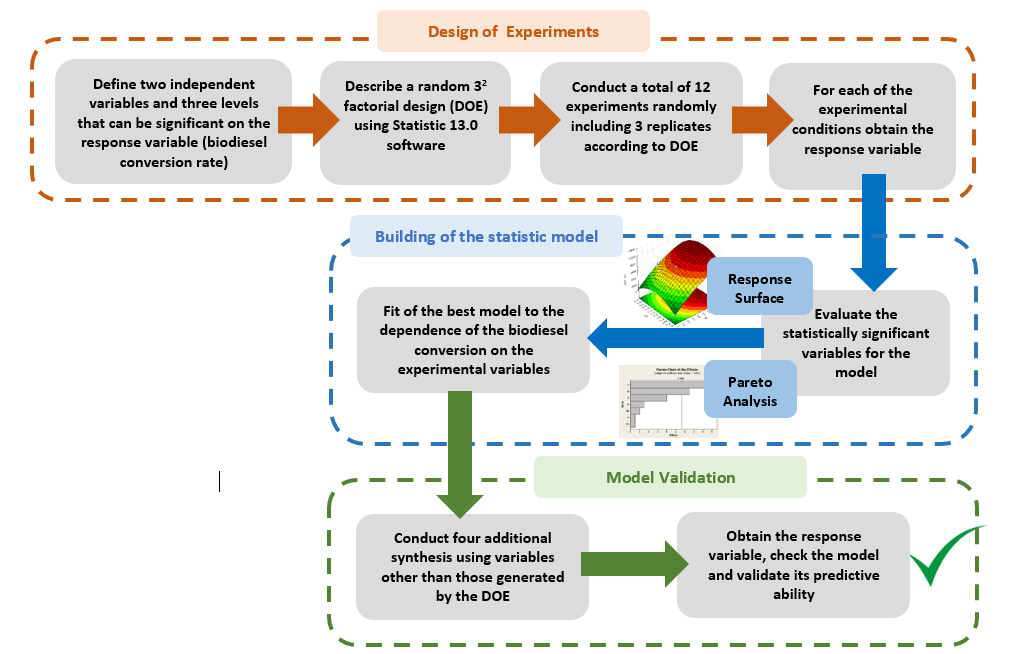
Q-2.3 - Given your choice of technique, briefly discuss an advantage and a potential practical limitation of using it to determine the yield of biodiesel. SI-3-Themes of Students’ SeminarsList of seminar themes assigned to student groups, G1 to G8. Some references were offered on these themes; the students did more literature search.Introduction to diesel engine fuels:Group 1- How does the diesel engine work? What is biodiesel (BD)? Discuss economic aspects (Brazilian and global) and environmental impact of the use of diesel (petroleum) and BD as fuels.Use of renewable material in the production of BD:Group 2- Discuss the most important properties of starting materials, and the relationship between chemical structures of BD and its performance as a fuel (discuss cetane number).Group 3- Choose two Brazilian oils that will be used to obtain BD, and an alcohol (methanol, or ethanol) and justify your choices.Note: as candidates, exclude the following oils: soybean, canola, corn, sunflower and palm oil.Mechanistic aspects of the BD production reaction:Group 4- Show the mechanisms of BD production reactions under homogeneous conditions, using Brønsted-Lowry and Lewis acid catalysts.Group 5- Show the mechanisms of BD production reactions under homogeneous conditions, using basic Brønsted-Lowry and Lewis type catalysts.Group 6- Show the mechanisms of BD production using heterogeneous acid- and base catalysts.BD Analytics and Performance:Group 7- Discuss the use of chromatographic methods (gas chromatography, HPLC) in the determination of the structure/yield of the BD.Group 8- Shows the use of non-chromatographic methods of determining the BD yield. Discuss instrumental methods for determining the quality/performance of BD, compared to diesel oil.SI-4-Tables and Figures
SI-3-Themes of Students’ SeminarsList of seminar themes assigned to student groups, G1 to G8. Some references were offered on these themes; the students did more literature search.Introduction to diesel engine fuels:Group 1- How does the diesel engine work? What is biodiesel (BD)? Discuss economic aspects (Brazilian and global) and environmental impact of the use of diesel (petroleum) and BD as fuels.Use of renewable material in the production of BD:Group 2- Discuss the most important properties of starting materials, and the relationship between chemical structures of BD and its performance as a fuel (discuss cetane number).Group 3- Choose two Brazilian oils that will be used to obtain BD, and an alcohol (methanol, or ethanol) and justify your choices.Note: as candidates, exclude the following oils: soybean, canola, corn, sunflower and palm oil.Mechanistic aspects of the BD production reaction:Group 4- Show the mechanisms of BD production reactions under homogeneous conditions, using Brønsted-Lowry and Lewis acid catalysts.Group 5- Show the mechanisms of BD production reactions under homogeneous conditions, using basic Brønsted-Lowry and Lewis type catalysts.Group 6- Show the mechanisms of BD production using heterogeneous acid- and base catalysts.BD Analytics and Performance:Group 7- Discuss the use of chromatographic methods (gas chromatography, HPLC) in the determination of the structure/yield of the BD.Group 8- Shows the use of non-chromatographic methods of determining the BD yield. Discuss instrumental methods for determining the quality/performance of BD, compared to diesel oil.SI-4-Tables and Figures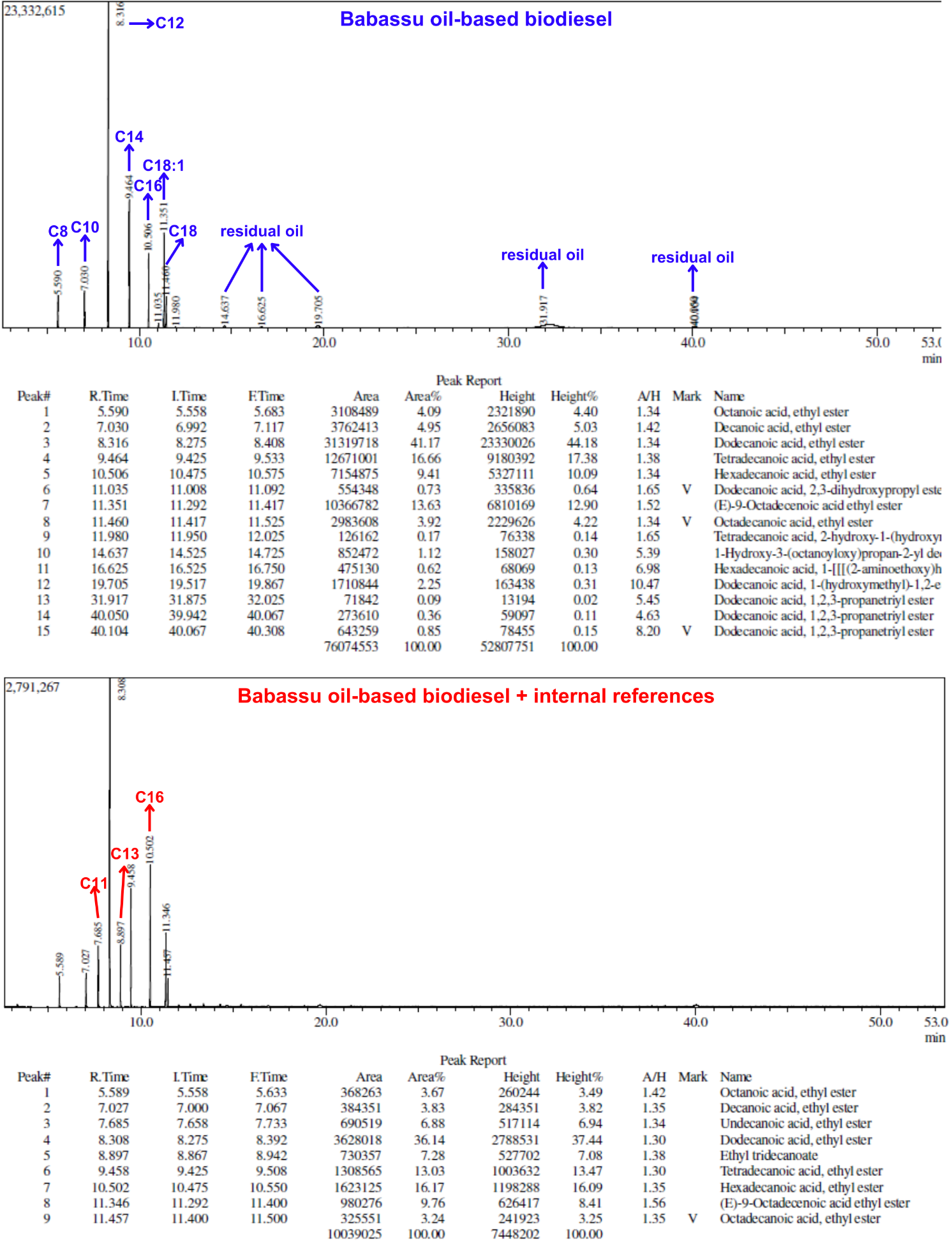
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML