-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2023; 11(2): 20-26
doi:10.5923/j.jlce.20231102.02
Received: Jul. 20, 2023; Accepted: Aug. 4, 2023; Published: Sep. 12, 2023

Education for Sustainable Development for High School Students: Synthesis of Biodiesel from an Amazon Region Oil (Babassu)
Nicolas Keppeler1, Narciso Rodrigo S. Vagula1, Maria Helena Zambelli2, Raquel N. Guimarães3, Victor Martins de Carvalho4, Omar A. El Seoud1
1Institute of Chemistry, the University of São Paulo, São Paulo, SP, Brazil
2Humboldt College, São Paulo, SP, Brazil
3Prof. Luiz Gonzaga Righini High School, São Paulo, SP, Brazil
4Waldorf Micael College, São Paulo, SP, Brazil
Correspondence to: Omar A. El Seoud, Institute of Chemistry, the University of São Paulo, São Paulo, SP, Brazil.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

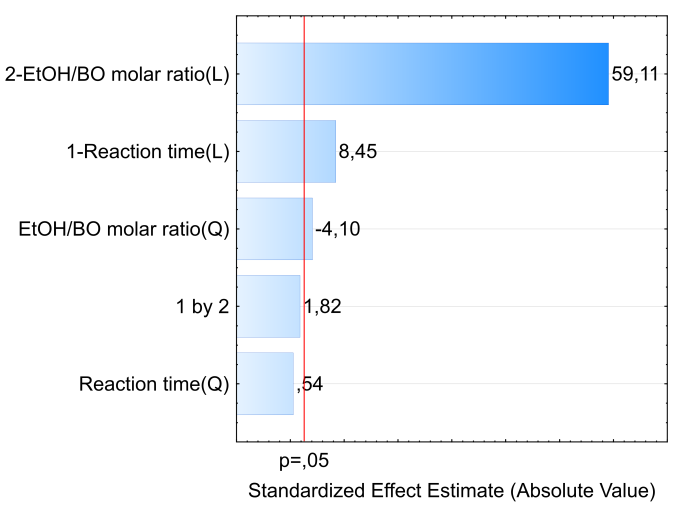
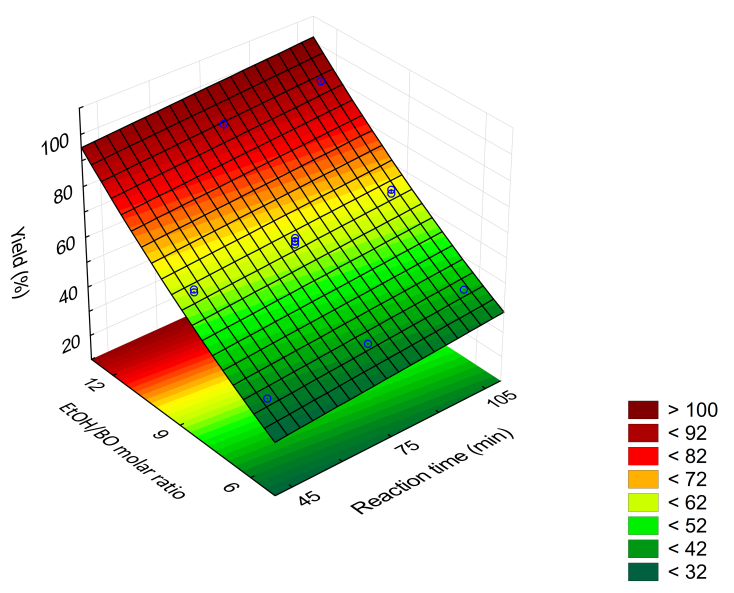
We introduced education for sustainable development to sixty high school (HS) third-year students, via a three-stage collaboration project between HSs and the Institute of Chemistry, University of São Paulo (ChemUSP). The general theme of the project is biofuels, with emphasis on biodiesel (BD). In the first stage, the students answered a questionnaire on important aspects of biofuels, including synthesis, and environmental impact. After discussing the results of this questionnaire, the students were informed (in the HSs) about the activities they will do at ChemUSP. During their visit to the latter, they synthesized BD from bioethanol and an Amazon region vegetable oil, babassu oil (BO), using a base catalyst (sodium ethoxide, C2H5ONa). The experimental variables were reaction time (45 to 105 minutes) and the molar ratio of bioethanol/babassu oil (χEtOH/BO; 6-12). We used design of experiment to generate a table, where the fourteen experiments of the three HSs were carried out in a random order. While doing the experiments, the students determined acid numbers of fresh-, and recycled vegetable oils, and visited the Central Analytical Laboratory of ChemUSP. We subjected the combined results of all students to statistical analysis using a commercial software. The Pareto chart, the 3D response surface and the corresponding correlation (BD yield versus experimental variables) showed that the BD yield depends on χEtOH/BO much more than on reaction time, within the ranges of the studied variables. These results were discussed with the students at the HSs, after their visit to ChemUSP. A second questionnaire was then given, now focusing on BD. The result showed greater awareness of relevant aspects of the subject, including the oils employed in the industrial process to obtain BD; transesterification of oils with bioethanol; environmental, and economic impact of using BD. The students evaluated this project positively because of its green chemistry approach (use of renewable feedstocks; use of chemometrics), because of their active participation in the chemistry laboratory (development of experimental skills), and their contact with ChemUSP.
Keywords: Education for sustainable development, Biofuels, Biodiesel, Green chemistry principals, Transesterification, Viscosity, Statistics
Cite this paper: Nicolas Keppeler, Narciso Rodrigo S. Vagula, Maria Helena Zambelli, Raquel N. Guimarães, Victor Martins de Carvalho, Omar A. El Seoud, Education for Sustainable Development for High School Students: Synthesis of Biodiesel from an Amazon Region Oil (Babassu), Journal of Laboratory Chemical Education, Vol. 11 No. 2, 2023, pp. 20-26. doi: 10.5923/j.jlce.20231102.02.
Article Outline
1. Introduction
- The United Nations Educational, Scientific and Cultural Organization (UNESCO) has been the leading agency for implementation of the Decade of Education for Sustainable Development (ESD; 2005-2014); the Global Action Program on ESD (2015-2019), and is now responsible for the implementation of ESD for 2030. Among other goals, these programs give “learners of all ages the knowledge, skills, values and agency to address interconnected global challenges including climate change, loss of biodiversity, unsustainable use of resources, and inequality” [1]. Regarding climate change, the (adverse) contribution of burning fossil fuels to the emission of greenhouse gases [2], in particular CO2, is established beyond doubt [3]. The key role of education in combating this global problem has been recognized; actions are being implemented, especially by United Nations agencies [4].Recently, we have started a program between High Schools (HSs) and the Institute of Chemistry of the University of São Paulo (ChemUSP) to promote the connection between these educational institutions. These apparently separate organizations perform the same function, and are, in fact, not only interrelated, but overlap [5,6]. The strategy of our program is to implement activities that start by discussing a project in the HS (in the present case, biodiesel, BD); doing the experiment in the undergraduate laboratory of ChemUSP (base-catalyzed synthesis of BD), and then analyze the data obtained in the HS, see Scheme 1 below. Our approach uses “learning by doing”, an active learning methodology, based on experience to assimilate concepts through actions. It also encourages the student to take decisions (where appropriate) and draw conclusions from their own results. In the present ESD project, we emphasize the significance of biofuels, particularly BD, in the context of green chemistry. Additionally, the students were introduced to chemometrics to show the relative importance of the two experimental variables studied, namely, the molar ratio of bioethanol/babassu oil (χEtOH/BO) and reaction time.
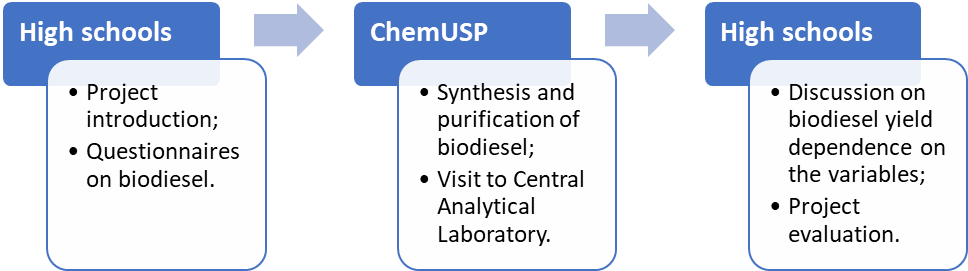 | Scheme 1. A flow diagram of the activities carried out in this project, both at high schools and at the Institute of Chemistry of the University of São Paulo (ChemUSP) |
 | Figure 1. Photographs of the high school students at the ungraduated laboratory of ChemUSP |
2. Experimental
2.1. Materials
- Commercial absolute ethanol (EtOH) 99.8% was from LabSynth, SP, and babassu oil (BO) was from COPPALJ oil extraction company, Maranhão, Brazil. The sodium ethoxide catalyst was freshly prepared before the experiment of each school, by dissolving clean sodium metal in absolute EtOH. The metal dissolution was carried out in an Erlenmeyer flask, protected with a Drierite-containing drying tube. The concentration of the base was determined before each HS visit by titration with standardized HCl solution; the final base concentration was adjusted to 0.152 mol L−1. Recycled frying oil was obtained from EcoABC, São Paulo. The material necessary for the experiments includes: 10 mL burettes, pipettes, Erlenmeyer flasks, semi-analytical balance, hotplates with magnetic stirring, borosilicate glass cylindrical oil baths containing ethylene glycol (EG); red spirit-filled immersion thermometers, Luer lock glass syringes, and separation funnels.
2.2. Procedures
2.2.1. Determination of the Acid Number (AN) of Babassu Oil and of Recycled Frying Oil
- Weigh ca. 5 g of fresh BO, and/or 1g of the recycled oil into separate 125 mL Erlenmeyer flasks. To each flask, add 50 mL of ethanol and 3-5 drops of phenolphthalein indicator solution. Using magnetic stirring and 10 mL burette, titrate each oil solution with alcoholic KOH solution (0.050 mol L−1) until the solution changes its color from colorless to (persistent) light pink. Calculate the oil acid number using Equation 1.
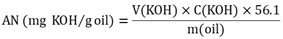 | (1) |
2.2.2. Synthesis of Babassu Oil-Based Biodiesel
- Place the provided EG heating bath (containing a magnetic bar) on a hotplate with magnetic stirring. Place the (hotplate + EG bath) on a laboratory scissor jack. Insert a thermometer into the EG and begin the (stirring + heating) of the EG until 95 ± 5°C. Concurrently, introduce a magnetic stirring bar into a 3-necked, 125 mL round-bottom flask; mark the flask with the groups´ name (G1, G2, etc). Using the indicated burette and a glass syringe, introduce the volumes of BO, and then the EtOH as indicated in Table 1 below. Once the reagents were added, close the three "necks" of the flask with the provided polypropylene stoppers.
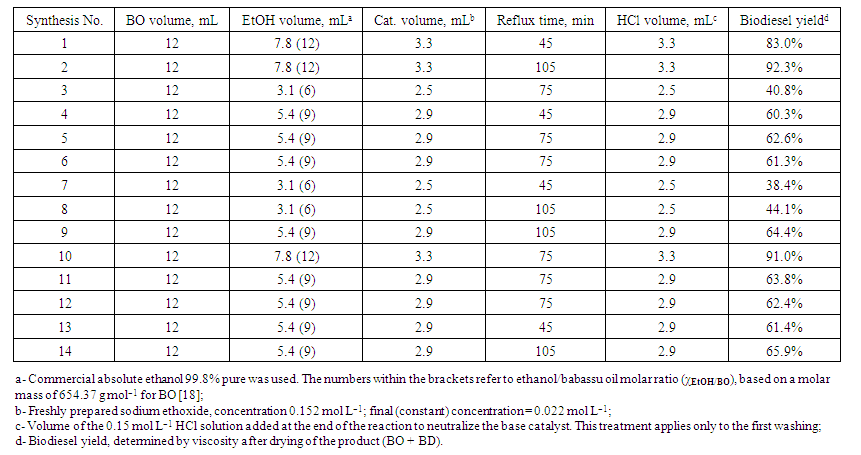 | Table 1. Synthesis of biodiesel by the high school students from babassu oil (BO) and ethanol (EtOH), using a sodium ethoxide/EtOH solution as a catalyst |
2.2.3. Calculation of the Biodiesel Yield from Viscosity Measurement
- In our previous article [6], we conducted a study on biodiesel yield determination by viscosity. The preparation of mixtures of BO and authentic BD from babassu oil was carried out using 5 mL burettes. Mixtures were prepared by varying mass percentages of authentic BD (15%, 30%, 45%, 60%, 75%, and 90%). The viscosities of the (BO + authentic BD) mixtures, were determined using the Brookfield rheometer. We obtained Equation 2 through regression analysis, using a linear fit (R² = 0.997). Based on these data, the students were able to calculate the biodiesel yields from viscosity measurements data.
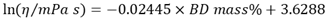 | (2) |
2.3. Hazards
- Ethanol poses hazards of flammability and toxicity. BO is combustible; sodium ethoxide-, and HCl solutions are corrosive. There are no significant hazards in this project that require precautions other than wearing personal protective equipment. All liquid wastes were collected and sent to the safety division of ChemUSP.
3. Results and Discussion
3.1. Relevance of the Project
- As stated in Introduction, the project is on base-catalyzed synthesis of BD, see Scheme 2.
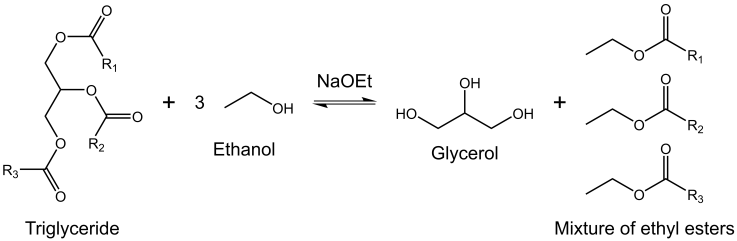 | Scheme 2. General scheme for transesterification of a triglyceride with ethanol in the presence of base catalyst, sodium ethoxide (NaOEt) |
3.2. Preparation in High School for ChemUSP Visit
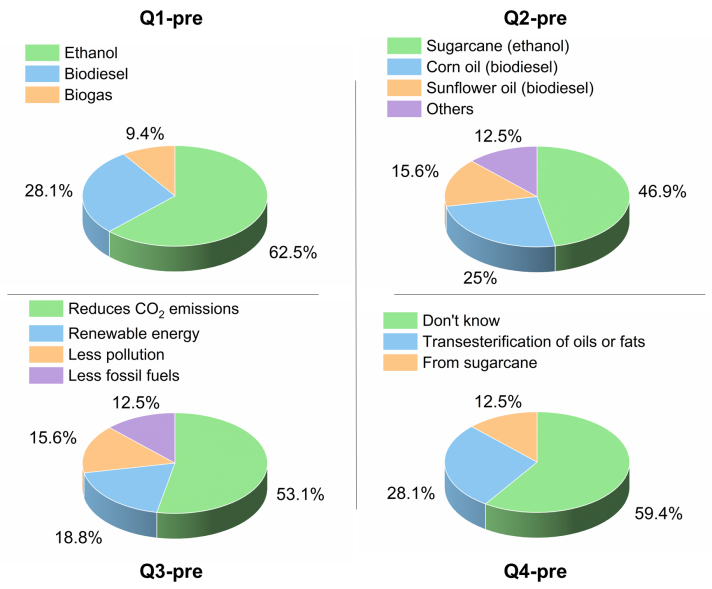
- After we informed the students about the HS-ChemUSP project, see Scheme 1, we gave a pre-visit questionnaire on aspects of biofuels, including:Q1-pre: Give examples of commercial biofuels;Q2-pre: What are the starting materials for the biofuels that you mentioned?;Q3-pre: Give two environmentally important effects resulting from using biofuels;Q4-pre: Do you know how biodiesel is commercially produced?A summary of the students´ answers to these questions is shown in Figure 2. The answers to Q1-pre were expected because ethanol alone was introduced in Brazil as a biofuel for passenger cars in 1975, and flex-fuel cars that run on gasoline, ethanol, and any mixture of these two fuels were introduced locally in 2003 [14]. Regarding Q2-pre, we were surprised by the percentage of answers that indicated the use of corn-, and sunflower oils for making BD because soybean oil is, by far, the main vegetable oil employed to make BD in Brazil, 71.2% in 2021 [15]. The answer to Q3-pre shows the awareness of the students about the (positive) environmental impact of using biofuels instead of petroleum-based fuels. The answers to Q4-pre reflect the fact that oils and fats are discussed only scantly during their chemistry course. This project, therefore, also fills an information gap about oils/fats and practical aspects of biofuels.
3.3. Activities at ChemUSP
- During all activities in the undergraduate laboratory of ChemUSP, the students of each school were accompanied by at least five persons, including, their teacher, two graduate students, one staff member from ChemUSP, and the laboratory technician. The 60 participating students were divided into 14 groups, each of four or five; they carried out the experiments in 3 different days, over a three-week period. These groups used different experimental conditions (of time and χEtOH/BO) that we generated using the Statistica software. The activities at ChemUSP involved: synthesis, purification and (partial) drying of BD; determination of the acid number of BO and that of a recycled frying oil, and a visit to CAL.For safety, we closely supervised the steps of the whole experiment, for details of the experiments see Table 1. These steps included: adjusting the temperature of the heating bath; assembly of the glassware; introduction of BO, EtOH and the catalyst (sodium ethoxide/EtOH) into the reaction flask; reflux of EtOH for the required time (45 to 105 minutes), cooling and washing of BD with NaCl solution, partial drying of BD. Because oil/water phase separation is relatively slow, we told the students no to dwell on mass of the produced BD, or its visual aspect after drying with MgSO4, clear or slightly turbid. There were no accidents, except breaking one red spirit-filled immersion thermometer! During the EtOH reflux time, the students did two activities: determination of AN of fresh BO and a recycled frying oil, and a visit to CAL, in groups of 8 each, where they were introduced to analysis by GC/MS and HPLC. The visit to ChemUSP ended when the students turned in their BD samples. These were further dried by heating under reduced pressure (vacuum oven). We measured the viscosities of the samples, and the results were sent back to the schools, along with a calibration curve of viscosity x BD mass% (in mixtures of BO plus authentic BD) [6].
3.4. Activities in the Schools after the Visit to ChemUSP: Project Conclusion and Evaluation
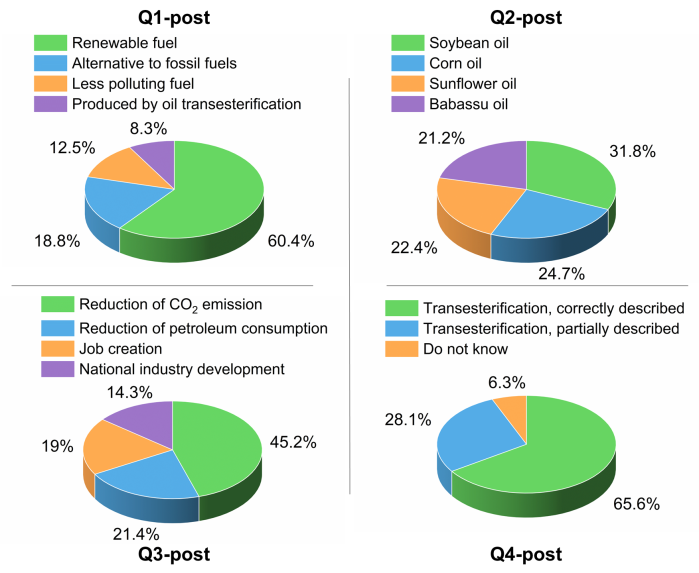
- After the visit to ChemUSP, the professors in the schools gave a post visit questionnaire Q-post, that is a modified version of Q-pre, with focus on BD. The answers to Q1-post are now focused on BD; the vegetable oils mentioned in Q2-post now include BO, because they used this oil at ChemUSP; the answers to Q3-post and Q4-post show greater awareness of the environmental impact of using diesel oil-BD blends instead of pure diesel oil, and knowledge of the industrial process for obtaining BD, respectively (see Figure 3).
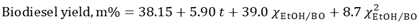 | (3) |
4. Conclusions
- ESD was introduced to students of three different HSs by using a hand-on approach, that involved pre- and post-activities in the HSs. The questionnaires given before and after the visit to ChemUSP showed an increased awareness of the students about the socioeconomic and environmental impacts of using diesel oil-BD blends. The activities at ChemUSP involved synthesizing BD, and contact with advance analytical instrumentation at CAL. It increased the interests of the students in green chemistry, especially as applied to solve an environmentally important problem, viz., reduction of greenhouse gas emission. This project shows applications of the principles of green chemistry: use of renewable feedstocks; use of statistics to achieve better yields [25]. We recommend its use due to the environmental relevance of BD, its simplicity, low cost, safety of the experimental part, and because it brings the school closer to the university.
ACKNOWLEDGEMENTS
- We are grateful to the students who participated in this project. We thank Professor Dr. Pedro V. de Oliveira of ChemUSP for his support and encouragement, Professor Priscila Teixeira Rivero (Luiz G. Righini HS) for her help, Alexandre S. Guarezemini (ChemUSP), Flavio Crispim (Humboldt College) for their competent technical support in the undergraduate laboratory, Angelica Maria S. de Oliveira and Francenilda C. P. Ciferi for their help with visits logistics. We acknowledge FAPESP (grant 2014/22136-4) and CNPq (grants 306108/2019-4; 141853/2019-0) for research fellowships for O. A. El Seoud and N. Keppeler, respectively.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML