-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2023; 11(2): 15-19
doi:10.5923/j.jlce.20231102.01
Received: Jul. 8, 2023; Accepted: Aug. 4, 2023; Published: Aug. 12, 2023

The Preparation of meso-Stilbene Dibromide: Scaffolding Green Chemistry Principles via an Electrophilic Addition Reaction
Justin Bindewald, Gina Dobiesz, Isabel Medrano-Cervantez, Kate Moody, Tyler Rockey, Joseph C. Sloop, Jessica Taza, Cynthia M. Woodbridge
School of Science and Technology, Georgia Gwinnett College, Lawrenceville, GA, USA
Correspondence to: Joseph C. Sloop, School of Science and Technology, Georgia Gwinnett College, Lawrenceville, GA, USA.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

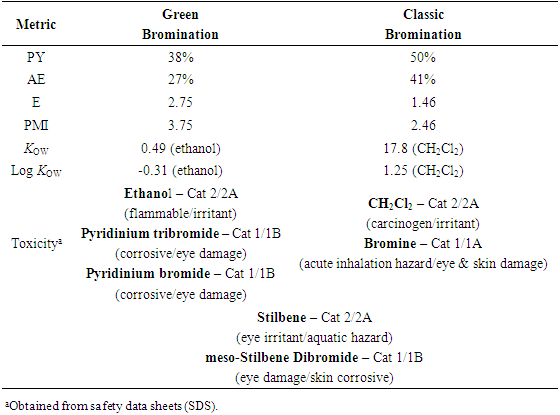
To better understand how green chemistry principles may be applied to a simple electrophilic addition reaction, students in the Green and Sustainable Chemistry course (CHEM 4000) at Georgia Gwinnett College were introduced to the bromination of E-stilbene in a scaffolded approach which culminated with the conduct of an experimental comparison of the classic bromination of E-stilbene using Br2 in CH2Cl2 and the green bromination of E-stilbene using pyridinium tribromide in ethanol. Student teams isolated the target meso-stilbene dibromide product and compared the processes using the green reaction metrics of percent yield (PY), atom economy (AE), waste metric (E-factor), process mass intensity (PMI), solvent suitability (Kow, log Kow) and hazard classes of reactants, products and by-products. The classic bromination was determined overall to be a more efficacious reaction than the green bromination based on the PY, AE, E-factor, and PMI. On the basis of Kow, log Kow and the hazard classes of reactants, products and by-products, the green bromination was determined to be less hazardous than the classic bromination.
Keywords: Atom economy, Waste metric, Process mass intensity, Kow,E-stilbene, Meso-stilbene dibromide
Cite this paper: Justin Bindewald, Gina Dobiesz, Isabel Medrano-Cervantez, Kate Moody, Tyler Rockey, Joseph C. Sloop, Jessica Taza, Cynthia M. Woodbridge, The Preparation of meso-Stilbene Dibromide: Scaffolding Green Chemistry Principles via an Electrophilic Addition Reaction, Journal of Laboratory Chemical Education, Vol. 11 No. 2, 2023, pp. 15-19. doi: 10.5923/j.jlce.20231102.01.
Article Outline
1. Introduction
- As part of a continuing initiative to incorporate green chemistry principles throughout the chemistry curriculum, faculty in the Department of Chemistry at Georgia Gwinnett College (GGC) developed the Green and Sustainable Chemistry course [1]. The American Chemical Society (ACS) guidelines for ACS certified chemistry programs lists green chemistry and sustainability as critical requirements for accreditation. [2] Progress in implementation of green chemistry in undergraduate chemistry curricula over the last two decades has helped enhance laboratory safety, drive facility improvements and enable faculty to infuse courses with environmentally responsible reaction alternatives. [3,4] The overarching goals of the course were to (1) expose students to the twelve Green Chemistry Principles, (2) develop student skills in the use green chemistry metrics to analyze chemical processes for their sustainability, and (3) apply these metrics to case studies in which green chemical synthesis methods and sustainable processes have been successfully employed. As part of the course design, the instructors selected Etzkorn’s text [5], which provides case studies to illustrate each of the twelve green chemistry principles. Based on the high impact practice of scaffolding on learning in chemistry [6,7], instructors then incorporated a well-known addition reaction, the bromination of 1,1'-[(E)-1,2-Ethenediyl] dibenzene, commonly known as E-stilbene, to scaffold throughout the course lessons in order to illustrate how green chemistry principles and specific green reaction metrics may be applied to achieve a more benign process. See Scheme 1.
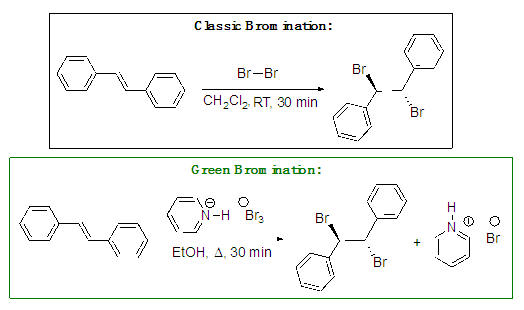 | Scheme 1. Classic and Green Meso-Stilbene Dibromide Syntheses |
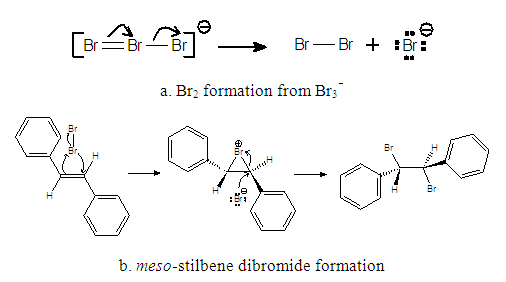 | Figure 1. E-Stilbene Bromination Mechanism |
|
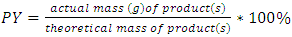 | (1) |
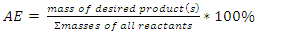 | (2) |
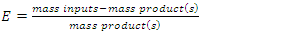 | (3) |
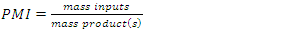 | (4) |
2. Materials and Instrumentation
2.1. Materials
- a. Experimental Instructions (adapted from the on-line Laboratory Text – Chemistry 2211K/2212K).b. E-stilbene, bromine, pyridinium tribromide, methylene chloride and ethanol were purchased from Fisher Scientific Chemical company and were used as purchased.
2.2. Instrumentation
- a. Melting points were obtained on an SRS Digimelt MPA160 melting point apparatus and were uncorrected.b. IR Spectra were collected on a Thermo Scientific Nicolet iS5 FTIR (600-4000 cm-1 range).
3. Experimental
- Students selected their lab team members. One team opted to perform the green bromination while the other team chose the classic bromination.
3.1. Green Bromination of E-Stilbene
- Chemicals used: E-Stilbene (Fisher Scientific, CAS 103-30-0), pyridinium tribromide (Fisher Scientific, CAS 39416-48-3), 96% ethanol (Fisher Scientific, CAS 64-17-5). All chemicals were used as purchased. Equipment used: stir/hotplate, sand bath, 10 mL round bottom flask, condenser, magnetic stir bar.Safety notes: a. Reaction must be carried out under the benchtop snorkel hoodlet.b. Students must use gloves and safety goggles while carrying out the experiment.For mole table: Use a 1:1.67 molar ratio of E-Stilbene: pyridinium tribromide. [12] This molar ratio was selected to ensure that a slight excess of the brominating agent was present during the reaction. E-Stilbene: brominating agent ratios for this reaction as high as 1:1.90 have been reported. [8] Procedure: A 10 mL round bottom flask equipped with a stir bar was charged with 6.0 mL ethanol and E-Stilbene (0.205g, 1.14 mmol) and placed in the sand bath. The solution was stirred to ensure dissolution and warmed to 50°C. Then, pyridinium tribromide (0.350 g, 1.90 mmol) was added to the warm, stirring solution in small portions over a five-minute period. A condenser was affixed to the flask and the mixture stirred for 30 min. After 30 minutes, the solution was allowed to cool to room temperature, and then placed in an ice bath to maximize crystal formation. The white crystalline product was isolated using vacuum filtration and the crystals washed with 3 x 5 mL aliquots of cold ethanol. The crystals were then dried using a Fries lamp and the percent yield and m.p. of the meso-stilbene dibromide were determined. Yield: 0.148 g (38%); melting range 239-242°C, lit. [14], m.p. 237°C. IR (cm-1): 3029 (Ar-H), 3021 (Ar-H), 1497(Ar C=C), 1454 (Ar C=C), 690 (C-Br). [15]
3.2. Classic Bromination of E-Stilbene
- Chemicals used: E-Stilbene (Fisher Scientific, CAS 103-30-0), freshly prepared 1.0 M solution Br2 in CH2Cl2, (Br2, 99.5%, obtained from Thermo Scientific, CAS 7726-95-6), CH2Cl2, (HPLC grade, Fisher Scientific, CAS 75-09-02). Equipment used: stirplate, 10 mL round bottom flask, magnetic stir bar.Safety notes: a. Reaction must be carried out under the benchtop snorkel hoodlet.b. CH2Cl2 is a carcinogen, students must use gloves and safety goggles while carrying out the experiment.c. The Br2/CH2Cl2 solution is hazardous; students must use caution when handling.For mole table: Use a 1:1.5 molar ratio of E-Stilbene: Bromine. Procedure: A 10 mL round bottom flask equipped with a stir bar was charged with E-Stilbene (0.200 g, 1.11 mmol) and 5.0 mL of dichloromethane (DCM). The mixture was stirred to ensure dissolution. Using a disposable pipette, 1 M bromine solution (1.65 mL, 1.65 mmol) (in DCM) was added slowly over a period of two minutes to the reaction mixture. The reaction mixture was then stirred at room temperature for 30 minutes. Upon completion, the reaction flask was chilled in an ice bath for 10 minutes. The white crystalline product was isolated via vacuum filtration. The product was washed with 3 x 5 mL portions cold water to remove any color from the crystalline material. The crystals were then dried using a Fries lamp and the percent yield and m.p. of the meso-stilbene dibromide were determined. Yield: 0.189 g (50%); melting range 238-241°C, lit. [14] m.p. 237°C. IR (cm-1): 3030 (Ar-H), 3023 (Ar-H), 1496 (Ar C=C), 1451 (Ar C=C), 688 (C-Br). [15]
3.3. Post Experimental Activities – Worksheet Completion and Discussion
- Instructors encouraged teams to work throughout the experimental process on the worksheet as the synthesis was underway. Following isolation of the product and cleanup, the student teams completed the worksheet, which is available as supplemental material [13]. Each team determined several metrics for their reaction which illustrated the “greenness” of the processes. The metrics included PY, AE, E-factor, PMI, Kow, log Kow and Toxicity ratings for the reactants, solvents, products and by-products. The results are found in Table 2. The worksheets were collected by the instructors for assessment as a lesson quiz.
|
4. Laboratory Experience Impact on Student Understanding of Green Chemistry Principles
- Following the laboratory exercise, students were asked to reflect on whether they viewed the experiment as a valuable part of the course. Students unanimously responded that they enjoyed the hands-on, real-world application of green chemistry principles. When asked if they would like to have additional experiments incorporated into the course, they indicated that they would. In the end of course survey, students gave similar positive responses to the bromination reaction scaffolding approach.
5. Conclusions
- This marks the first year that the Green and Sustainable Chemistry course was offered at GGC. The scaffolding of green and classic electrophilic E-stilbene bromination reactions throughout the course provided students with a means to utilize green chemistry principles and parameters to compare simple reactions. Course outcome assessment data suggests that students applied green chemistry metrics successfully as part of a live laboratory in which they compared two types of brominations. Overall, student surveys show that the use of the bromination scaffold was as a vehicle for learning how green chemistry principles may be applied to make reactions more environmentally responsible.
ACKNOWLEDGEMENTS
- The instructors thank the GGC Department of Chemistry for the classroom and laboratory support during the course.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML