-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2023; 11(1): 11-14
doi:10.5923/j.jlce.20231101.03
Received: May 31, 2023; Accepted: Aug. 2, 2023; Published: Aug. 12, 2023

Studies of Tethering Atom Effect in Cobalt-catalyzed Alkyne/Nitrile Cyclization Reactions towards the Synthesis of Tetrahydronaphthyridines with Shown Significant Activities against Mycobacterium Tuberculosis
Ya Zhou, Daniel Encarnacion, Connor Sullivan, Vivian Tam
Department of Chemistry and Physics, Salem State University, Salem, MA, United States of America
Correspondence to: Ya Zhou, Department of Chemistry and Physics, Salem State University, Salem, MA, United States of America.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Nitrogen-containing small heterocyclic molecules have drawn great attention in the design of biologically active molecules in pharmaceutical industry. We have been interested in the synthesis of nitrogen- containing 5,6,7,8-tetrahydro-1,6-naphthyridines. Recently, several molecular libraries of this 6,6-membered bicyclic tetrahydronaphthyridines have been synthesized and have shown significant activities against Mycobacterium Tuberculosis. We noticed that these reported microwave-promoted cobalt-catalyzed intermolecular alkyne/nitrile [2+2+2] cyclization reactions gave only poor to modest yields. These modest reaction yields are suspected to be caused by a probable unfavorable chelation of the nitrogen atom of the cyclization precursor with the cobalt catalyst. This chelation may retard the formation of the key cobaltacycle reaction intermediate. In this study, we investigate the effect of different tethering atoms such as nitrogen, carbon and oxygen in this cyclization reaction using a 5,6-membered bicyclic model study system. We observe that lower cyclization reaction yields are obtained from the nitrogen-tethered precursors compared to the other precursors. Our next step is to elucidate the reason for low-yielding using the real 6,6-membered bicyclic system. We plan to protect the nitrogen atom in cyclization precursor with different protecting groups to see if the protected nitrogen-tethered precursors can provide higher alkyne/nitrile [2+2+2] cyclization reaction yields.
Keywords: Tetrahydronaphthyridine, Mycobacterium Tuberculosis, High atom economic synthesis, Cobalt-catalyzed, Alkyne/Nitrile, [2+2+2] Cyclization, Tethering atom effect
Cite this paper: Ya Zhou, Daniel Encarnacion, Connor Sullivan, Vivian Tam, Studies of Tethering Atom Effect in Cobalt-catalyzed Alkyne/Nitrile Cyclization Reactions towards the Synthesis of Tetrahydronaphthyridines with Shown Significant Activities against Mycobacterium Tuberculosis, Journal of Laboratory Chemical Education, Vol. 11 No. 1, 2023, pp. 11-14. doi: 10.5923/j.jlce.20231101.03.
Article Outline
1. Introduction
- Small polyfunctionalized heterocyclic molecules have attracted great attention in the design of biologically active compounds in the drug discovery process as well as in the isolation and structural identification of biological macromolecules [1-4]. Many natural and unnatural heterocyclic molecules inhibit the progression of the cell cycle through binding to the proteins required for cell division. In this way, heterocyclic small molecules can also be employed as tools to understand cell cycle events. Among these heterocycles, nitrogen-containing heterocycles have received increasing attention in the past two decades. The nitrogen-containing heterocycle is not only able to readily accept or donate a proton, but it can also easily establish a diverse weak interactions with a variety of enzymes and receptors in biological targets through hydrogen bindings, dipole-dipole interactions, hydrophobic effects, van der Waals forces and π-stacking interactions. FDA databases reveals nearly 75% small-molecule drugs contain a nitrogen heterocycle [5].Fully aromatized naphthyridines are nitrogen-containing heterocyclic molecules defined as a group of six isomeric heterocyclic systems with two fused pyridine rings having different relative positioning of the nitrogen atoms (Figure 1). This general class of compounds has received great interest from organic chemists because of the exceptionally broad range of biological activities displayed by some of its members [6-7].
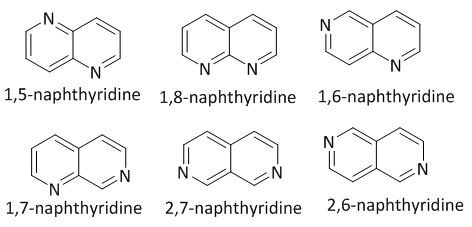 | Figure 1. Naphthyridine isomers |
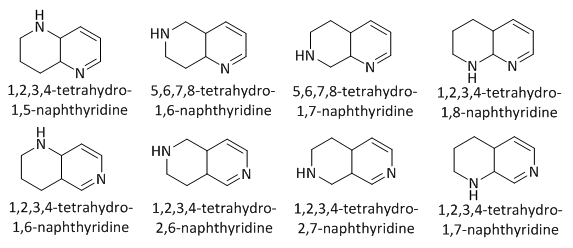 | Figure 2. Tetrahydronaphthyridine isomers |
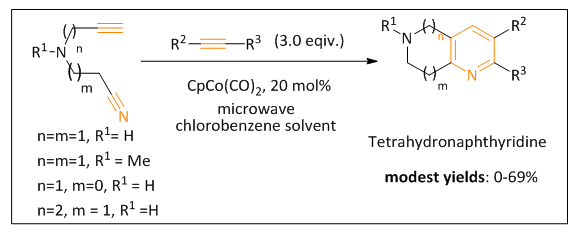 | Figure 3. Modest yields observed in the reported intermolecular [2+ 2+ 2] alkyne/nitrile cyclization reactions |
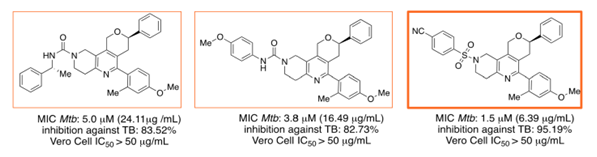 | Figure 4. Three 5,6,7,8-tetrahydro-1,6-naphthyridines with inhibition against Mycobacterium Tuberculosis |
2. Experimental
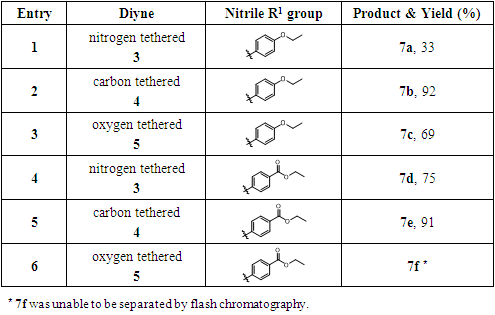
- The reported modest yields of the [2+2+2] cyclization reactions are suspected to be caused by a probable unfavorable chelation of the nitrogen tethering atom of the cyclization precursor alkynyl nitrile with the cobalt catalyst, which may retard the formation of the key cobaltacycle reaction intermediate. To test our hypothesis, we synthesized a series of cyclization precursors with different tethering atoms as a model study to investigate the effects of different tethering atoms in this type of cyclization reactions. Even though these model reactions only provided the 5,6-membered bicyclic products 6a-6d and 7a-7f as shown in Scheme 1 and Scheme 2, not the 6,6-membered bicyclic products 5,6,7,8-tetrahydro-1,6-naphthyridines, these studies still helped us to understand the tethering atom effect in this type of cobalt-catalyzed [2+2+2] cyclization reactions. Our future plan is to synthesize the nitrogen tethered precursor of the 5,6,7,8-tetrahydro-1,6-naphthyridine and protect the precursor with different nitrogen protecting groups. Using the real system, we can exam if the protected precursors can provide tetrahydronaphthyridine products in better yields.
2.1. Experimental Design
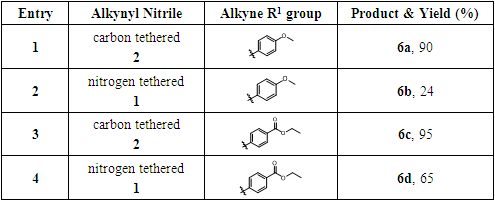
- The alkynyl nitrile precursor 1 with nitrogen tether and the percussor 2 with carbon tether were prepared according to the literature procedures [8]. The cyclization reactions of these precursors with another alkyne partner were tested (Scheme 1). These two precursors 1 and 2 were reacted with the same set of alkyne partners respectively. One alkyne partner has an electron donating group, while the other one has an electron withdrawing group. These reaction results are discussed in Result and Discussion section.
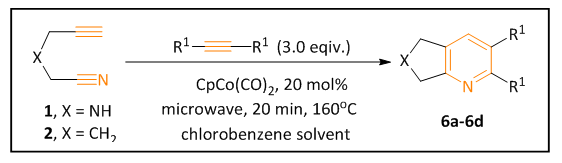 | Scheme 1 |
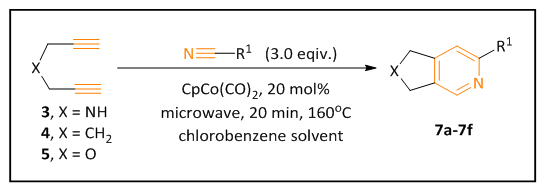 | Scheme 2 |
2.2. Procedure
- General procedure of the intermolecular microwave- promoted cobalt-catalyzed alkyne/nitrile [2+2+2] cyclization reaction: all reactions were run in sealed 10 mL thick-walled microwave pressure tubes (CEM Corporation) purged with nitrogen in a CEM Discover SP microwave synthesizer (Figure 5). For the cyclization reactions in Scheme 1, a chlorobenzene solution of alkynyl nitriles 1 or 2 (1.0 eq) and alkynes (3.0 eq) were added into the microwave tube, then the catalyst CpCo(CO)2 (0.2 eq, 20 mol%) was added into the mixture. For the cyclization reactions in Scheme 2, a chlorobenzene solution of diynes 3, 4 or 5 (1.0 eq) and benzonitriles (3.0 eq) were added into the microwave tube. The resulting solution was subjected to microwave irradiation at 300 W, 160°C for 20 min with stirring.
 | Figure 5. CEM Discovery SP microwave synthesizer, microwave pressure tube, celite plug and flash chromatography |
3. Results and Discussion
- The results of the cyclization reactions in Scheme 1 were summarized in Table 1. As we expected, the intermolecular cyclization reactions of the carbon-tethered alkynyl nitrile 2 with alkyne partners provided products 6a and 6c in much better yields (90% in entry 1 and 95% in entry 3) compared to the nitrogen-tethered alkynyl nitrile 1 (6b, 24% in entry 2; 6d, 65% in entry 4). Furthermore, we observed the similar trends in both the electron donating alkyne substate (entries 1 and 2) and the electron withdrawing alkyne substrate (entries 3 and 4).
|
|
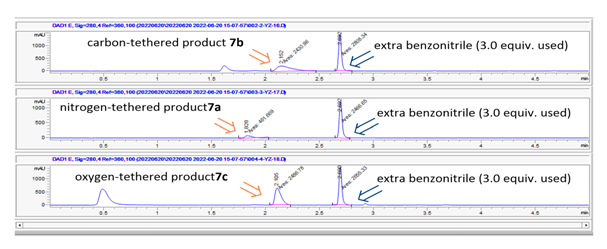 | Figure 6. Crude LC-MS data for entries 1-3 in Table 2 |
4. Conclusions
- This alkyne/nitrile intermolecular [2+2+2] cyclization reaction is a high atom economic reaction. It provides us an efficient way to synthesize this rarely addressed biologically active 5,6,7,8-tetrahydro-1,6-naphthyridines. The results obtained from these studies supported our hypothesis that the previously reported modest yields of the cyclization reactions were related with the existence of the nitrogen tethering atom. After replacing the nitrogen tethering atom of the reaction precursor with carbon or oxygen tethering atoms, our model cyclization reactions proceeded in higher yields. Our continuing effort will be focusing on synthesizing the nitrogen-tethered processor of 5,6,7,8-tetrahydro-1,6- naphthyridine and further improving the cyclization reaction yield in real system by protecting the nitrogen atom with different protecting groups.
ACKNOWLEDGEMENTS
- We thank Salem State University for funds and consumables associate with this work. Special thanks to Jessica Christel for ordering, providing us with the materials and equipment.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML