-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2023; 11(1): 7-10
doi:10.5923/j.jlce.20231101.02
Received: Apr. 3, 2023; Accepted: May 4, 2023; Published: May 12, 2023

Synthesis of the Moderately Basic Soap Utilizing Fatty Acid Waste
Jilian Amurao, Tyler Brack, Estelle Nuckels, Renat Khatmullin
Department of Natural Sciences, Middle Georgia State University, Cochran, GA 31014, United States of America
Correspondence to: Renat Khatmullin, Department of Natural Sciences, Middle Georgia State University, Cochran, GA 31014, United States of America.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Cost-effective and green chemistry methods are appealing in both teaching and research laboratories. We present how fatty acid waste mixtures can be recycled in a subsequent soap-making experiment. Colligative Properties experiments that utilize fatty acids as both solute and solvent in General Chemistry 2 lab courses work well. However, the drawback of this experiment is the laborious clean-up associated with fatty acids adhering to the glassware. Fatty acids, or mixtures thereof, are also used as reactants in soap-making experiments. We recycle the fatty acid mixture in a separate soap synthesis reaction. The methodology adopted in this approach reduces both chemical waste and, more importantly, promotes greener methods in teaching undergraduate chemistry laboratories.
Keywords: Soap, Colligative Properties, Freezing Point Depression, Melting Point, Fatty Acids, Green Chemistry, Vernier LabQuest
Cite this paper: Jilian Amurao, Tyler Brack, Estelle Nuckels, Renat Khatmullin, Synthesis of the Moderately Basic Soap Utilizing Fatty Acid Waste, Journal of Laboratory Chemical Education, Vol. 11 No. 1, 2023, pp. 7-10. doi: 10.5923/j.jlce.20231101.02.
Article Outline
1. Introduction
- Ideal experiments in undergraduate teaching labs utilize nonhazardous inexpensive reagents that generate minimal waste. The experiments selected in teaching labs need to illustrate the course content well with a sure outcome, even if the students deviate from the prescribed procedure. We can tie together two experiments that function very well as illustrations so that the second experiment remedies the difficult clean-up of the first experiment. A main topic in the second semester general chemistry course is colligative properties, looking at changes in vapour pressure, melting point, boiling point, or osmotic pressure. Changes in vapour pressure or osmotic pressure measurements are more challenging to complete than experiments involving melting or boiling point change. Saltwater solutions are commonly used as they are non-hazardous, easy to rinse and also include experience with the van’t Hoff factor [1-4]. However, it does require large quantities of salt, leading to a salty residue that can be found on counters for weeks to come. In addition, problems with forming a supercooled liquid in a freezing point depression experiment can frustrate both student and instructor. A colligative properties experiment involving fatty acids as solute and solvent remains a simple, nonhazardous option. [3] Using stearic acid as a solvent, students can determine its melting point via a temperature probe and graphical analysis when a sample is merely heated in a hot water bath. After adding an unknown solute (palmitic, myristic, or lauric acids) to stearic acid solvent, students melt the mixture and use graphical analysis to determine the mixture’s melting point. The change in melting point is used to identify the unknown solute. If graphs are overlaid, students are able to visually see the depression of the melting point, something that helps solidify the freezing point depression concept in their minds.
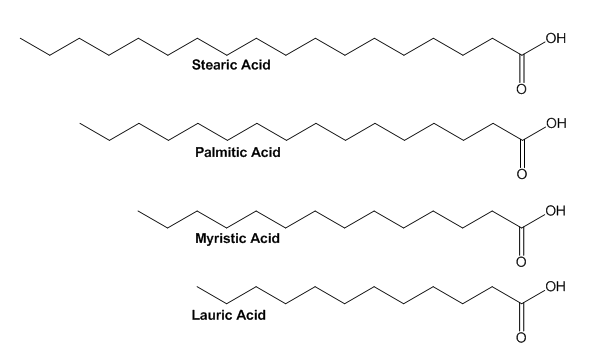 | Figure 1. Fatty acids selected for Colligative Properties experiment |
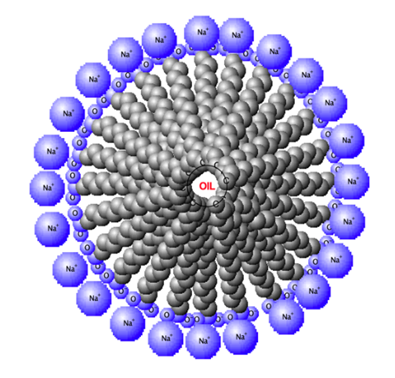 | Figure 2. Formation of the soap micelle with the oil being in the center of the micelle |
2. Experimental Part
- Materials needed: Supplied in the supplemental part of the manuscript.
2.1. Colligative Properties Experiment
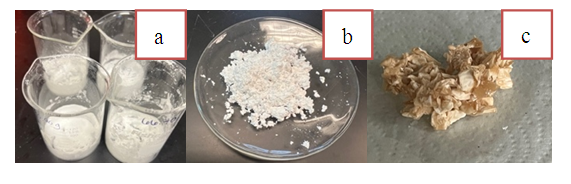
- Though unnecessarily large for this portion of the experiment, students should obtain approximately 8 grams of stearic acid in a pre-weighed 150mL beaker. The size of the beaker is based on the needed reagents for the recycling of the product in the subsequent soap synthesis experiment. The amount of stearic acid need not be precise at this time; however, the mass of the empty beaker must be known. The beaker containing the stearic acid should be suspended in a hot water bath using a 400mL beaker or larger on a hot plate. (Figure 3a). The water should be allowed to warm above 80°C, but not boil, as condensation from the water vapours will skew calculations. Once the stearic acid melts (above 69.3°C), the resulting liquid should be allowed to warm slightly. After that, remove the beaker from the warm water bath. With constant stirring, the fatty acid mixture will solidify. During this process, students record the temperature at consistent intervals using LabQuests with temperature probes or a comparable product. After the stearic acid freezes, the temperature will drop, and students can stop recording data. Students can determine the melting point of the stearic acid using the average value found from the shaded constant temperature region of their graph (Figure 3b).
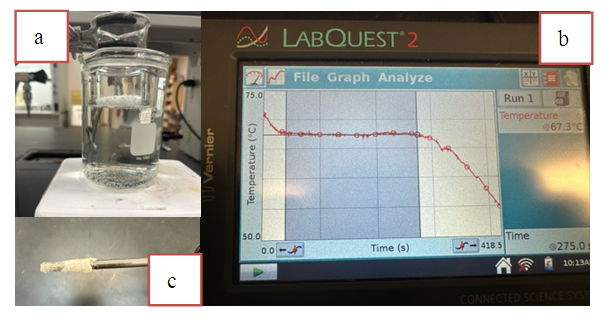 | Figure 3. a) Colligative Properties setup using the fatty acid mixture. b) Temperature vs. time graph. c) Adherence of fatty acid to the probe |
2.2. Soap Synthesis Experiment
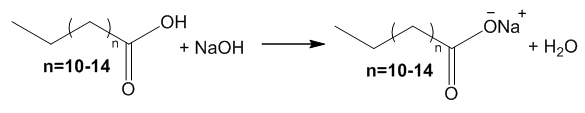 | Figure 4. Soap Formation Equation |
3. Results and Discussion
3.1. Colligative Properties
- The data collected should be saved during the experiment. If the experimentally determined freezing point is not as expected (69.3°C, within the expected error of the machine), it is likely that the students did not select the appropriate portion of the pure stearic acid graph (Figure 3b). Students also have difficulty determining the melting point of the mixture. If multiple trials show inconsistent results, students should revisit the graph data and extrapolate straight lines for the slopes before and after freezing. The intersection of these lines will provide a more accurate measurement of the mixture's freezing point. Overlapping all the graphical data provides a visual representation of freezing point depression. The sample data is provided in the supplementary part of this manuscript. Students should have recorded the melting point of both pure stearic acid and the mixture. They will need to calculate the mass of stearic acid remaining in the beaker and the mass of unknown acid added to the beaker. The molality of the mixture can be found from the change in melting point and the known Kf value of stearic acid (4.5°C/m). After the conversion of the mass of solvent (stearic acid) to kilograms, students can determine the moles of solute and, subsequently, the molar mass. The calculated molar mass typically only has about two significant figures as the change in melting point is limited by the precision of the temperature probe. Students should be reminded when identifying the unknown acid. Students must write the mass of the empty beaker on the side before finishing the experiment, as the information is necessary and helpful for the subsequent soap making reaction.
3.2. Synthesis of Soap
- The fatty acid waste consisted of a mixture of Stearic acid (major component) with either lauric, myristic, or palmitic acid (minor components). Students determine the melting points of the fatty acid mixtures prior to soap-making, which are in the expected broad range of 60-70°C. The formed soap can be characterized as a white powdered substance with no distinct scent and poor water solubility. The pH tests confirmed that the formed soap's pH exceeded the pH of 12. Although students were not expected to calculate the exact product yield, we expected reaction to yield soap quantitatively. The melting point of the synthesized soap samples was reported to be in the range of 230-260°C supporting the formation of the product.
4. Conclusions
- Designing undergraduate chemistry lab experiments that produce low to no waste remains one of the goals of teaching undergraduate chemistry. It also aligns with the twelve principles of green chemistry. In the pairing of a general chemistry colligative properties lab with an organic chemistry soap making lab, we are able to reduce waste and ease student clean-up while still retaining student laboratory experiences. The colligative properties experiment effectively demonstrates the freezing point depression of stearic acid due to the addition of either palmitic, myristic or lauric acid. The resulting waxy fatty acid readily reacts with sodium hydroxide in a reaction generating sodium stearate mixed with an additional sodium salt, confirmed via melting point. While the fruit juice concentrates lower the pH, it limits the efficacy of the resulting soap. Upon neutralization or curing of the sodium stearate mixture, the nonhazardous materials are effectively used twice, lowering the cost of materials.
ACKNOWLEDGEMENTS
- We would like to acknowledge the STEM grant committee of Department of Natural Sciences and Middle Georgia State University for providing the resources and funding to complete the research work.
Supplementary Information
- The complete lab handouts can be accessed using the following link: will be provided later in the journal upon acceptance of manuscript.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML