-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2023; 11(1): 1-6
doi:10.5923/j.jlce.20231101.01
Received: Dec. 22, 2022; Accepted: Jan. 30, 2023; Published: Feb. 7, 2023

Sustainable Fuels for High School Students: Synthesis of Biodiesel from an Amazon Region Oil (Babassu)
Omar A. El Seoud1, Nicolas Keppeler1, Maria Helena Zambelli2
1Institute of Chemistry, the University of São Paulo, São Paulo, SP, Brazil
2Colégio Humboldt, Av. Engo. Alberto Kuhlmann, São Paulo, SP, Brazil
Correspondence to: Omar A. El Seoud, Institute of Chemistry, the University of São Paulo, São Paulo, SP, Brazil.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

We describe here a four-stage project on the synthesis of a sustainable fuel (biodiesel, BD) from an Amazon region oil, namely, babassu oil (BO). The project was carried out both at Humboldt high school (project discussion and evaluation) and at the Institute of Chemistry, the University of São Paulo, ChemUSP (properties of BO; synthesis of BD). The students were asked to discuss aspects (origin, economic and environmental) of (petroleum-based) diesel oil and BD; suggest a candidate oil/fat from the Amazon region and an alcohol for the synthesis of BD, and discuss experimental variables that bear on biofuel yield. The students discussed this assignment in class, as well as what they are going to do during the third (experimental) stage of the project, carried out at ChemUSP. The students determined the acid- and saponification number of BO, synthesized BD using an optimized experimental protocol, prepared mixtures of (BO + authentic BD) and were introduced to gas chromatography. The students evaluated the project positively because of the socioeconomic- and environmental relevance of BD, its production from an Amazon region oil, and their contact with ChemUSP. We recommend this project for high school students because of its simplicity, safety, low cost, and because the school/university contact is beneficial for both sides.
Keywords: Babassu oil, Biodiesel synthesis, Acid-base titration, Transesterification, Catalysis, Chemical equilibria
Cite this paper: Omar A. El Seoud, Nicolas Keppeler, Maria Helena Zambelli, Sustainable Fuels for High School Students: Synthesis of Biodiesel from an Amazon Region Oil (Babassu), Journal of Laboratory Chemical Education, Vol. 11 No. 1, 2023, pp. 1-6. doi: 10.5923/j.jlce.20231101.01.
Article Outline
1. Introduction
- Biodiesel, BD, denotes the fuel composed of mixtures of fatty acid esters (usually methyl or ethyl) obtained by the transesterification of oils and fats. We developed a four-stage project for twelfth-grade high school students on the synthesis of BD, with emphasis on connecting theory to practice. In the first stage of the project, conducted at Humboldt school, we introduced some aspects of BD, including its economic importance (in 2021, Brazil consumed 5.57 billion liters of biodiesel) [1], some relevant properties of oils and fats (acid- and saponification number), and aspects of the reactions used to produce BD (esterification; transesterification; catalysis) [2]. We divided the seventeen participating students into 4 groups, each of 4 or 5, and gave them the following assignment for the next class, based on literature that we provided [3–6]: (i) discuss aspects (origin, economic and environmental) of (petroleum-based) diesel oil and BD [7]; (ii) suggest candidate oil/fat from the Amazon region [8]; and an alcohol (methanol, ethanol, and 1-propanol) to synthesize BD; (iii) discuss some experimental variables that affect the yield of BD [9]. Regarding this assignment, we explained that the transesterification reaction will be base-catalyzed; we asked the students to consider the availability/price of the starting materials.In the following class (project stage 2 in the school), the students discussed differences in origin and combustion products of diesel oil and BD. Because of CO2 fixation during plant growth, the contribution of BD combustion to the green-house effect is much smaller than that of diesel oil, in addition to being Sulphur-free, and emits less CO, CO2, nitrogen oxides and particulate matter [10,11]. The students also showed how BD is synthesized, chose an Amazon region oil (babassu oil, BO), and discussed briefly what they are expected to do during their visit to the Institute of Chemistry, the University of São Paulo (ChemUSP). In project stage 3, the four student groups visited ChemUSP, where they did the following experiments in the undergraduate laboratory, under our supervision: determined the acid- and saponification number of BO; prepared six mixtures of (BO + authentic BD) for viscosity measurement, and synthesized BO-based BD. The students visited the Central Analytical Laboratory (CAL) of ChemUSP where they were introduced to gas chromatography (CG), the technique most employed for the qualitative and quantitative analysis of BD [12]. Later, the instructor determined the viscosity of the above-mentioned (BO + authentic BD) samples, the four BD samples that the students prepared, and then sent the results to the students who calculated the BD yields of their experiments. The last stage of the project was carried out at the school, where the four groups presented their data and evaluated the project. We attribute the students´ highly positive evaluation to: the socioeconomic relevance of BD and the lower environmental impact of its combustion, relative to diesel oil; participation of the students in choosing the starting materials; their visit to ChemUSP, and the connection between the experimental part of the project and the theory they study (esterification, acid-base titration, and chemical equilibrium). We recommend this project for high school students because of its safety, low cost, and the simplicity of the equipment required. The project offers an opportunity to link experiment to theory, and enhances school/university interaction.
2. Experimental
2.1. Material
- Babassu oil (CAS 91078-92-1) was from Coppali, Lago do Junco, Maranhão, Brazil. Based on literature data [8], the molar mass of this BO was taken as 654.37 g mol-1. Commercial “anhydrous” bioethanol (CAS 64-17-5; hereafter designated as “ethanol”; 99.8 wt%), standardized HCl- (CAS 7647-01-0) and standardized KOH solutions (CAS 1310-58-3), diethylene glycol (CAS 111-46-6), NaCl (CAS 7647-14-5) and anhydrous MgSO4 (CAS 7487-88-9) were from Synth, São Paulo, all were used as received. An authentic sample of BD was synthesized by the instructor, by reacting BO with EtOH in the presence of H2SO4 catalyst as given elsewhere [13]. Based on CG analysis carried out by CAL, the purity of the authentic BD sample was 99.8%.
2.2. Equipment
- The students used Corning 6798-420D PC-420D digital stirring hotplate; Sorvall Legend X1R centrifuge (Thermo Scientific). The instructor dried the mixtures prepared in section 2.3.3 (BO + authentic BD), and the students´ BD samples (section 2.3.4) under reduced pressure using Fisher model 281A vacuum oven, and determined their viscosities using Brookfield model R/S-CPS cone-plate rheometer. The density was measured at 25C using Anton Paar DMA-4500 digital densimeter. The purity of the authentic BD sample was determined at CAL using Shimadzu model CG-msQP2010 gas chromatograph, equipped with 30m capillary column (BPX5, 5% phenyl methylpolysiloxane).
2.3. Procedures
2.3.1. Determination of the Acid Number (AN) of Babassu Oil
- Dissolve accurately weighed oil (ca. 10 g) in 75 mL of ethanol (EtOH) and add 0.5 mL of phenolphthalein indicator. Using a magnetic stirrer, titrate the solution using the furnished standardized ethanolic KOH solution (0.05 mol L-1). Additionally, perform a blank titration (using EtOH only). Calculate the value of AN of BO by the Eq. 1.
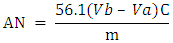 | (1) |
2.3.2. Determination of the Saponification Number (SN) of Babassu Oil
- Dissolve accurately weighted BO (ca. 1.5 to 2 g) in 25 mL of ethanolic KOH solution (0.5 mol L-1). Reflux the mixture for 1h to ensure saponification, characterized by a visually clear solution. Wash the condenser with ca. 10 mL of hot EtOH, add the washing to the saponification solution. Add 0.5 ml of phenolphthalein indicator solution, stir the solution magnetically, titrate the excess KOH present in the solution using the provided, standardized HCl solution (0.5 mol L-1). Additionally, perform a blank titration (using KOH solution only).Calculate the SN by Eq. 2 below:
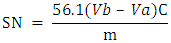 | (2) |
2.3.3. Preparation of Mixtures of BO + Authentic BD for BD Yield Determination
- Using 5 mL burettes, prepare 2 mL mixtures of (BO + authentic BD) containing 15, 30, 45, 60, 75, and 90 volume % of the provided authentic BD. For example, pipette into the provided tubes 0.3, 0.6, 0.9 mL, etc. of authentic BD and 1.7, 1.4, and 1.1 mL, etc. of BO. Mark the tubes and give them to the instructor. Calculate the mass % of authentic BD in these mixtures from the densities at 25°C of both components, 0.9176 and 0.8857 g mL-1, for OB and BD, respectively. Caution: BO is a viscous liquid that flows slowly. Consequently, it takes time for the meniscus to stabilize in the burette.
2.3.4. Synthesis of Babassu Oil-Based Biodiesel
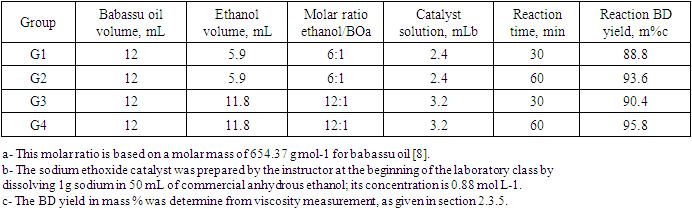
- Place the stirring hotplate on a laboratory jack; place the provided diethylene glycol (DEG) heating bath on the hotplate; introduce a thermometer (range 0-230°C) into the DEG, and start stirring/heating the liquid to 100 ± 5°C (set the heating power dial of the hotplate at 4). Parallelly, introduce a magnetic stirring bar into 125 mL 3-necked round bottom flask; use 25 mL and 10 mL burettes to introduce into the flask the volumes shown in Table 1 of BO and ethanol, respectively; close all necks with stoppers. Assemble the transesterification reactor above (not in contact with) the DEG bath, by inserting a reflux condenser provided with CaCl2 drying tube into the central neck of the flask. Using a 10 mL burette, introduce the provided catalyst solution (ethanolic sodium ethoxide) into a 10 mL addition funnel with a pressure equalizing arm (caution: the stopcock of the funnel should be closed); insert the funnel into one neck of the reaction flask.When the DEG temperature is reached, circulate water into the reflux condenser and use the laboratory jack to carefully raise the hot plate/DEG bath until the liquid in the 3-necked flask is below the DEG level. When ethanol starts refluxing gently, open the stopcock of the addition funnel to introduce the base catalyst solution at once, and start recording the reaction time, as listed in Table 1.
|
2.3.5. Calculation of the Biodiesel Yield from Viscosity Measurement
- This was carried out by the instructor and the students. The former determined the viscosities of the (BO + authentic BD) mixtures prepared by the students, as well as the viscosities of the BD samples furnished by the students after drying, using the Brookfield viscosimeter. Based on these data, the students calculated BD yields, vide infra.
2.4. Hazards
- There are no significant hazards in this project that require precautions other than wearing personal protective equipment. The aqueous waste was discarded by the safety division of ChemUSP.
3. Results and Discussion
- Scheme 1 shows the transesterification reaction, under acid- or base catalyzed reaction, the students used the latter (C2H5ONa/C2H5OH); the activities carried out by the students are shown in Scheme 2.
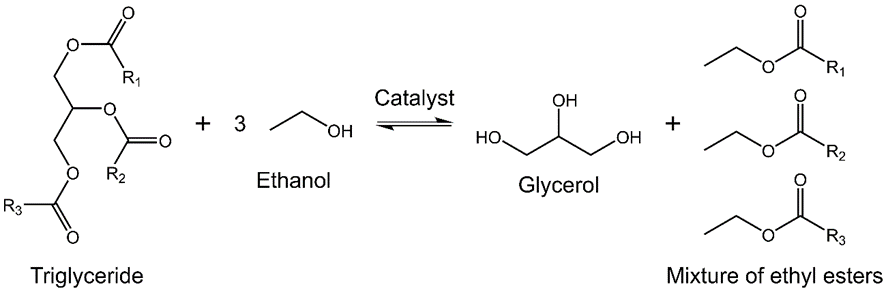 | Scheme 1. General scheme for transesterification of a vegetable oil (triglyceride) with an alcohol (usually methanol or ethanol) in the presence of acid- or base catalyst |
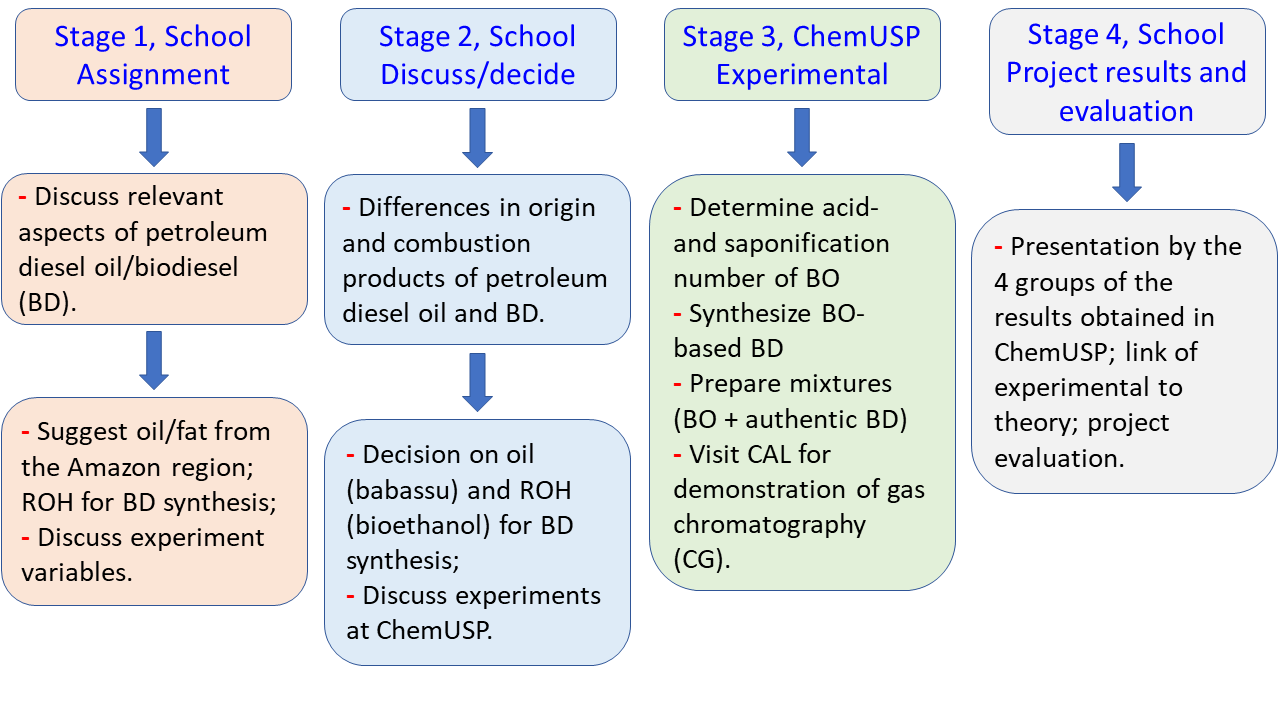 | Scheme 2. Activities carried out by the students at the school and ChemUSP during this project |
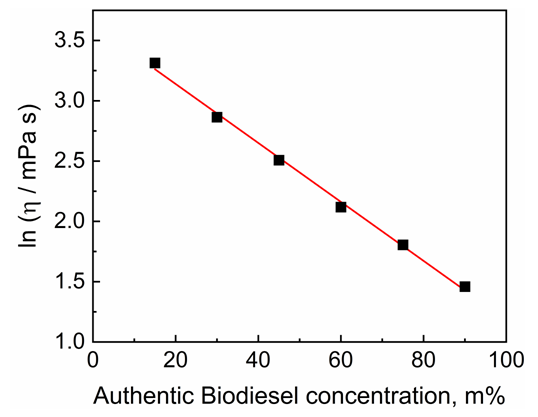 | Figure 1. Dependence of the viscosity (η) of mixtures (BO + authentic BD) on the mass% of the latter component in the mixtures |
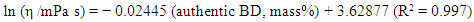 | (3) |
4. Conclusions
- It is important to introduce the subject of sustainable fuels in high school because of their socio-economic importance, lesser environmental impact, and because it will take years before diesel engines are partially, or totally phased-out [22]. Using oils and fats that are not intended for foods for making BD is critical because of the “food versus fuel” debate [23], which led to the increased use of waste cooking oils to make BD [24-26]. In the present project the students chose an Amazon region oil that is not used in foods because of its high saturated fatty-acid content, ca. 89.5% [8]. The students assembled a reactor, carried out a transesterification experiment, and separated the product in one four-hour period, thanks to our supervision/support and the experimental simplifications that we introduced. Additionally, the students were introduced to a “novel, sophisticated” analytical technique (CG) during their visit to CAL. We recommend this project to high-school students because of its simplicity, safety, low cost, the connection between experimental and theory, and because it represents an everyday situation, namely, manipulating a renewable material to produce BD, a fuel with less environmental impact than diesel oil.
ACKNOWLEDGEMENTS
- We are grateful to the students who participated in this project. We thank Professors Pedro V. de Oliveira and Shaker C. Farah (ChemUSP), Mrs. Melissa K. S. Ufer, Regina L. Vaz, Talita M. de Oliveira Silva (Humboldt high school) for their encouragement to do the project, Alexandre S. Guarezemini, Flávio Crispim and Guilherme B. Ranea Olivieri for their competent technical support in the undergraduate laboratory. We acknowledge CNPq (grants 306108/2019-4; 141853/2019-0) for research fellowships for O. A. El Seoud and N. Keppeler, respectively.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML