-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2022; 10(3): 59-66
doi:10.5923/j.jlce.20221003.03
Received: Nov. 9, 2022; Accepted: Nov. 30, 2022; Published: Dec. 6, 2022

Data-led Synthesis: An Inquiry-based Learning Project in Reaction Optimization with DigitalGlassware®
Matthew Barnes1, Linden Black1, George Cadman1, Aiden Cranney1, Jessica Giddins1, Ella Hamilton1, Henry Jones1, Christopher Marlow1, Eleanor Pomiankowski1, Hannah Simpson1, Francesca Slack1, Luka Vukoje1, Robbie Warringham2, David Pattison2, Andrew Nortcliffe1
1School of Chemistry, University of Nottingham, Nottingham, NG7 2RD, United Kingdom
2DeepMatter®, 38 Queen St, Glasgow, G1 3DX, United Kingdom
Correspondence to: Andrew Nortcliffe, School of Chemistry, University of Nottingham, Nottingham, NG7 2RD, United Kingdom.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Inquiry-based learning approaches to laboratory education begin to bridge the gap between expository laboratory experiments and the open-inquiry research environment. In collaboration with DeepMatter we have utilised their DigitalGlassware® software and DeviceX hardware in a student-led project to investigate reaction optimization to introduce students to the reproducibility problems faced by the chemical industry. Students recorded real-time data of a reaction using DeviceX and used this to design further reaction iterations to work towards a more reproducible, optimized reaction. Using DeviceX gave students a real-life insight into the value of data and data science in the future of chemistry.
Keywords: Upper-Division Undergraduate, Inquiry-based learning, Chemoinformatics, Laboratory Computing / Interfacing
Cite this paper: Matthew Barnes, Linden Black, George Cadman, Aiden Cranney, Jessica Giddins, Ella Hamilton, Henry Jones, Christopher Marlow, Eleanor Pomiankowski, Hannah Simpson, Francesca Slack, Luka Vukoje, Robbie Warringham, David Pattison, Andrew Nortcliffe, Data-led Synthesis: An Inquiry-based Learning Project in Reaction Optimization with DigitalGlassware®, Journal of Laboratory Chemical Education, Vol. 10 No. 3, 2022, pp. 59-66. doi: 10.5923/j.jlce.20221003.03.
Article Outline
1. Introduction
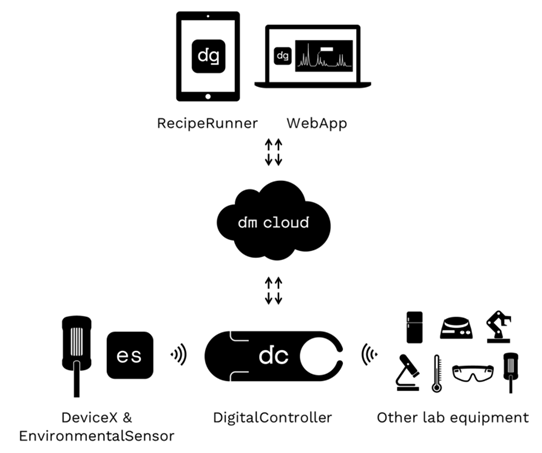
- The reproducibility crisis in Chemistry has been widely reported on and remains a key challenge for the future of the field. [1] Many reasons for irreproducibility have been debated and include flawed experimental methods more often than fraud. Over 80% of chemists report that they have failed to repeat an experiment from the literature and over 60% have failed to repeat one of their own experiments. [2] One of the key skills to ensure reproducibility is sufficient laboratory experience to apply chemical intuition to a reaction procedure and this lead to a successful outcome, [3–5] This experience is often limited to those who have practised at a postgraduate level or beyond and is a key focus for the application of machine learning in chemistry research. [6–8] One area where a lack of reproducibility can be directly associated to a lack of experience is in undergraduate teaching. This is not just a problem for chemistry, with other sciences reporting reproducibility flaws at the undergraduate level such as P-value hacking, [9] HARKing [10] and drawing conclusions from undersized samples. [11,12] Part of the undergraduate laboratory learning experience is to perfect fundamental techniques that are critical to successful science, and consequentially, reproducible results. But despite standardized training, often there is a variety of skill and practical chemical intuition across a similar cohort of students. Going forward to research-based learning this can result in experimental failure as the students face a steeper learning curve.One way in which reproducibility can be improved is using standard operating procedures and linking these to measurable outcomes in real-time. Allowing students to recognise how minor differences in an approach change the overall outcome, and to optimize their practise to perfect skills. Using this approach, relatively inexperienced scientists should be able to perform more complex experiments successfully with replicable outcomes. The use of real-time data analysis provides one of these measurable outcomes. The aim is that the outcome of a chemical reaction can be predicted based on its real-time reaction profile and how this compares to previous attempts. With the advent of new process analytical technologies to acquire this real-time data such as ReactIR and ReactNMR, [13–16] it is important to provide learning opportunities for undergraduate students in this area to enrich their learning experience and improve their employability.At the University of Nottingham students in the third-year of their MSci(Hons) degree in Chemistry or Chemistry with Medicinal and Biological Chemistry undertake mini-projects that give students the opportunity to plan and design their own experiments under guidance from an academic member of staff. [17] In this manner students exhibit higher-order cognitive skills, and this leads to a more valuable learning experience. The aim of this model is to bridge the gap between standard expository laboratory instruction and the open-inquiry model of a research environment. This approach has been successful and the outcomes of some of these projects have been published in the research literature. [17–19]In a pioneering collaboration between the University of Nottingham and DeepMatter® developed an inquiry-led project based on the use of DigitalGlassware® to optimise a chemical reaction. The aim of this project was to highlight the variation within student groups even when following a ‘recipe-style’ experiment and to challenge the students to design experiments to reduce this variability, and such improve reproducibility. This student-centred inquiry-based approach allows students to take responsibility for the design and direction of the project and develop the new ‘recipes’ to improve reproducibility using the DigitalGlassware® platform.DigitalGlassware® is an innovative cloud-based digital chemistry platform from DeepMatter® that allows users to capture and analyse a rich array of information about their chemical reactions. The platform records time course sensor data from the reaction flask against XML-structured experimental protocols (recipes) that contextualise the chemist’s actions in the lab. Outcomes such as yields and purity, and characterisation data such as chromatograms and spectra, can also be retained against the run record, improving analysis and interpretation capabilities. Coupling these rich run records with aggregated cloud based data presents opportunities for aggregated data analysis, machine learning and better understanding of the underlying chemical processes. DigitalGlassware® consists of several software and hardware components (Figure 1):
2. Experimental Model
- The reaction chosen for students to explore the synthesis of N-(1-naphthoyl)-4-methylbenzenesulfonohydrazide as reported in Organic Syntheses (Scheme 1). [20] This reaction requires the students to demonstrate several fundamental techniques essential for success in the organic chemistry laboratory but leaves some scope for interpretation of the procedure.
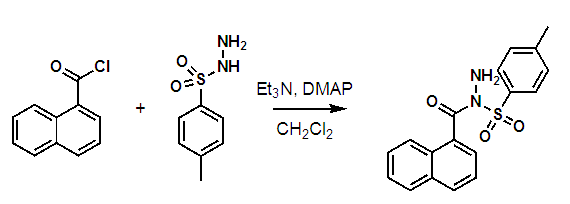 | Scheme 1. N-Sulfonylation reaction between 1-napthoyl chloride and p-toluenesulfonyl hydrazide |
2.1. Pedagogical Values
- These laboratory experiments have the following learning goals1. To introduce students to the use of real-time data collection using DigitalGlassware®.2. To allow students to reflect on their reactions and evaluate reaction outcomes and relate these to experimental procedures.3. To design new experimental procedures in response to collected data in an effort to optimise a reaction.4. To introduce students to the power of data science and its potential applications within Chemistry (including artificial intelligence and machine learning).The overall optimization of the reaction was not set as a goal for the project as more value was placed on the process and the decisions made by the students.
2.2. Hazards
- All experiments should be performed in well-ventilated fume hoods; suitable eye protection, hand protection (nitrile gloves) and a laboratory coat should be worn. Eye and skin contact, inhalation and ingestion of all chemicals should generally be avoided. 4-Dimethylaminopyridine is highly toxic by skin absorption, causes eye and skin burns, and all eye and skin contact and inhalation should be avoided. p-Toluenesulfonyl hydrazide is toxic if swallowed and flammable, do not use in a laboratory where there are naked flames. there is a risk of explosion if heated in a closed system. Dichloromethane, tetrahydrofuran, and acetonitrile should be used with special caution, since they may cause damage to the eyes, skin, brain, liver, lung, and central nervous. Dichloromethane is a known carcinogen. All solvents should be handled in a well-ventilated fume hood. Triethylamine and N,N-diisopropylethylamine are flammable liquids, cause skin burns and eye damage and are harmful if swallowed. Citric acid causes serious eye irritation. 1-Naphthoyl chloride is toxic by inhalation, causes eye and skin burns, and all eye and skin contact and inhalation should be avoided. Only use in a well-ventilated fume hood.
3. Results
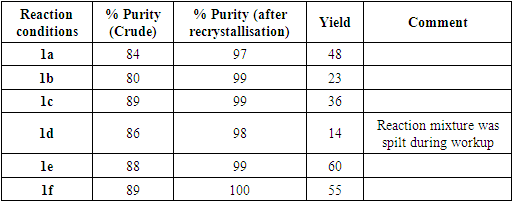
- In the first week, the students worked in pairs to each complete the reaction (pairs a-f) using the same procedure and following the recipe on the DigitalGlassware® platform. They were asked to work independently and to avoid conferring or comparing their experiment to the others; relying solely on the experimental procedure and their experience and experimental intuition. The reactions were monitored using DeviceX and students were able to monitor the real time data through the tablet. Each pair of students was able to prepare the sulfonamide successfully, although as expected there was significant variation in the overall yield (Table 1).
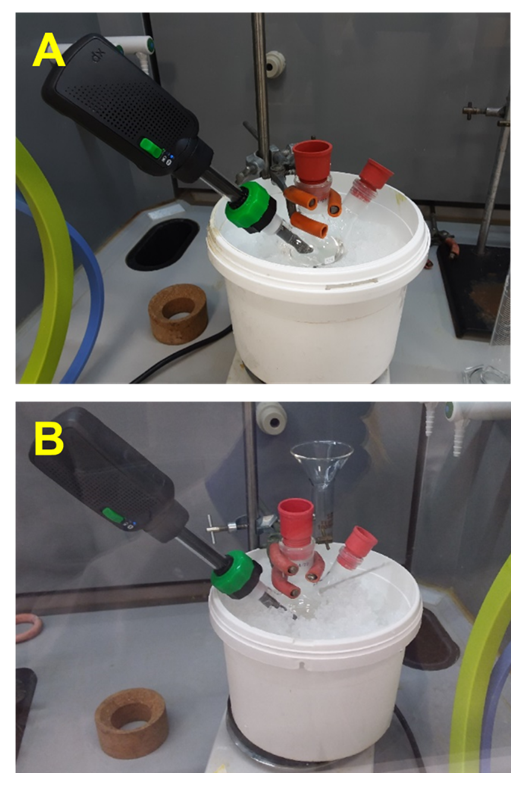 | Figure 3. Image notes taken by the students with the RecipeRunner tablet app used to capture the ice bath set-ups. Note the difference in immersion depth in A cf. B |
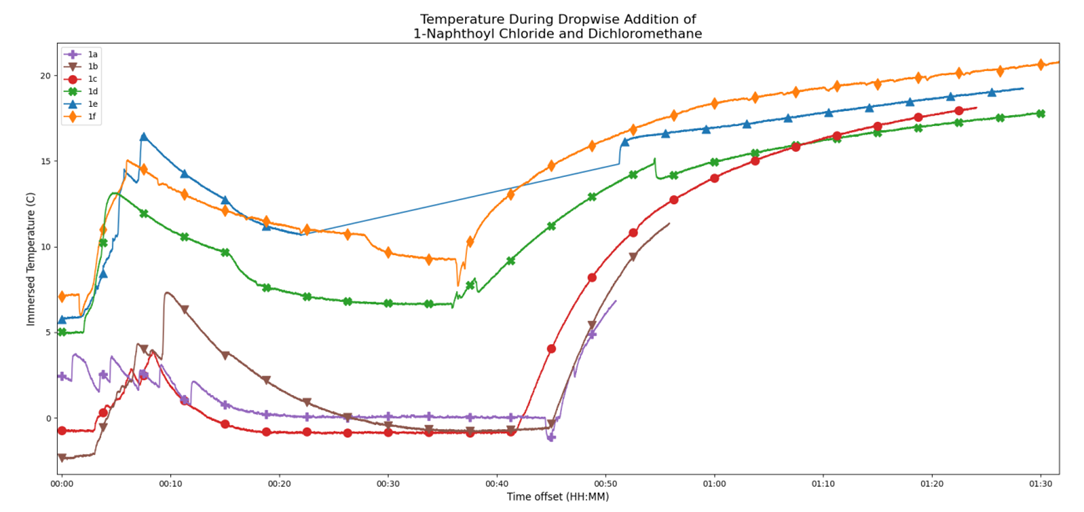 | Figure 4. An overlay of temperature profiles captured by the 6 groups during the addition of 1-napthoyl chloride/CH2Cl2 mixture (Reactions 1a-f) |
|
|
4. Discussion
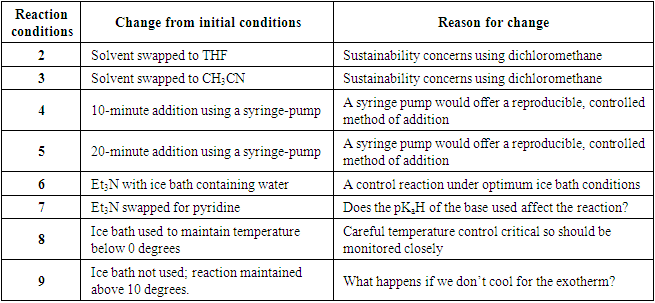
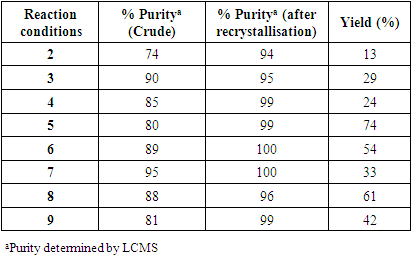
- We sought extensive informal feedback from the students through individual conversations throughout the project and after. They particularly appreciated working with technology and data to inform their decisions – recognising the global boom of data-based science and the importance of this for future employability. They also spoke favourably about working on an experiment that paralleled a real-life challenge – reproducibility and the input of scientists working at DeepMatter®. The following are quotes from the student authors taken from an evaluation survey of the project.• “I thoroughly enjoyed working with the equipment provided by DeepMatter®. Mainly because It was the first time I have worked with technology and data in chemistry.”• “Working with DeepMatter® on this project was very interesting; it highlighted to me the inconsistencies in the reporting of findings in the field of synthetic chemistry which ultimately lead to issues with reproducibility.”• “Although our work was cut short, I thought our findings were conclusive and supported our ideas of how we would optimize our reaction, none of which would have been possible without the data that DeviceX collected.”• “I think after I graduate, having some underlying knowledge and experience in this field will be inherently useful and will be something not many other undergraduates have.”• “Since using the DeepMatter® DeviceX I think using technology and software in the lab is almost essential. The amount of data that can be obtained is immeasurable compared to traditional methods.”• “The project allowed us to understand what was required for a successful optimisation and made us think of potential apparatus and techniques to optimise a reaction. Additionally, the idea of working with DigitalGlassware® that has never been used in an undergraduate lab before was very exciting.”• “We get an insight into what people are developing and how chemistry can be used outside of a lecture theatre.”• “Being able to monitor these endotherms or exotherms on a graph in real time (as well as the other parameters monitored by DeviceX) forces you to think more deeply about the chemistry that is happening as a result of each step of the method.”• “We’ve come across new techniques such as using a syringe pump or a dropping funnel, trying out different acids and bases, practicing a quench or a reverse quench and varying the temperature of the reaction.”• “I think DeepMatter®’s DigitalGlassware® would be a great tool to have access to in early-stage undergrad synthetic labs; as an undergraduate in a teaching lab there is a lot to think about, particularly as a first or second-year student, where many of the techniques and pieces of equipment you are using are new to you.”• Due to the timing of this project (February/March 2020) and the impact of COVID-19 restrictions, students were not assessed on this work. Had assessment taken place the ability of them to work well as a team would have been assessed and how the data they generated allowed them to make decisions in the lab. A laboratory report detailing the experiments undertaken and outputs of data from the DigitalGlassware® platform with a suitable written narrative of the discussions and decisions made would have identified successful completion of the learning goals. An oral presentation to supplement this would have allowed opportunity to explore student thinking and decision-making processes. • The overall expectation of students to fully optimise a reaction was not a pedagogical goal of this work, mainly due to the time restraints of the lab. However, for future applications of this project then a specific optimisation should be identified, giving students something to aim for in their work. The judicious choice of chemistry to investigate with students is also important, for example, reactions that are known to give multiple products or variations in diastereomeric / enantiomeric ratio are good examples to investigate due to additional important reaction outcomes, other than yield. One limitation of the work is the scale of the reaction suitable to be analysed. Due to the size of the DeviceX sensor, the tip containing the sensors must be submerged in solvent in order to gather accurate data, it is optimized to work in 100 mL or 250 mL round-bottomed flasks; microscale reactions would not be suitable to analyse with this equipment. Due to the timing of this project (February/March 2020) and the impact of COVID-19 restrictions, students were not assessed on this work. Had assessment taken place the ability of them to work well as a team would have been assessed and how the data they generated allowed them to make decisions in the lab. A laboratory report detailing the experiments undertaken and outputs of data from the DigitalGlassware® platform with a suitable written narrative of the discussions and decisions made would have identified successful completion of the learning goals. An oral presentation to supplement this would have allowed opportunity to explore student thinking and decision-making processes. The overall expectation of students to fully optimise a reaction was not a pedagogical goal of this work, mainly due to the time restraints of the lab. However, for future applications of this project then a specific optimisation should be identified, giving students something to aim for in their work. The judicious choice of chemistry to investigate with students is also important, for example, reactions that are known to give multiple products or variations in diastereomeric/enantiomeric ratio are good examples to investigate due to additional important reaction outcomes, other than yield. One limitation of the work is the scale of the reaction suitable to be analysed. Due to the size of the DeviceX sensor, the tip containing the sensors must be submerged in solvent in order to gather accurate data, it is optimized to work in 100 mL or 250 mL round-bottomed flasks; microscale reactions would not be suitable to analyse with this equipment.
5. Conclusions
- The DeepMatter® DigitalGlassware® platform is a user-friendly apparatus that has been successfully applied to an undergraduate project investigating reaction optimisation of a typical transformation carried out in industry. DeviceX collected real-time information about the reaction that was monitored via a tablet computer. Students were then able to use this data to develop new experimental procedures to investigate the outcome of the reaction. Through this project, students were able to experience first-hand the value of data in chemistry and how data science will continue to impact on practical chemistry. Student feedback was overwhelmingly positive with students highlighting the skills obtained and their value of them in future employability. Since the completion of this project, DeepMatter® has continued to work with researchers at the University of Nottingham in further developing projects that utilise the DigitalGlassware® and DeviceX technology to further expand the usability of this technology and some of these results have recently been published. [22]The data captured by the students and procedures (recipes) is available for download at https://github.com/deepmatterltd/dm_nortcliffe.
ACKNOWLEDGEMENTS
- We thank the University of Nottingham for funds and consumables associated with this work. Special thanks to Peter Morgan-Tansley for helping set up the DigitalGlassware® equipment at Nottingham.
References
| [1] | Bergman, R. G.; Danheiser, R. L. Reproducibility in Chemical Research. Angew. Chemie Int. Ed. 2016, 55 (41), 12548–12549. https://doi.org/https://doi.org/10.1002/anie.201606591. |
| [2] | Baker, M. 1,500 Scientists Lift the Lid on Reproducibility. Nature 2016, 533 (7604), 452–454. https://doi.org/10.1038/533452a. |
| [3] | Gomez, L. Decision Making in Medicinal Chemistry: The Power of Our Intuition. ACS Med. Chem. Lett. 2018, 9 (10), 956–958. https://doi.org/10.1021/acsmedchemlett.8b00359. |
| [4] | Moosavi, S. M.; Chidambaram, A.; Talirz, L.; Haranczyk, M.; Stylianou, K. C.; Smit, B. Capturing Chemical Intuition in Synthesis of Metal-Organic Frameworks. Nat. Commun. 2019, 10 (1), 539. https://doi.org/10.1038/s41467-019-08483-9. |
| [5] | Gibb, B. C. Chemical Intuition or Chemical Institution? Nat. Chem. 2012, 4 (4), 237–238. https://doi.org/10.1038/nchem.1307. |
| [6] | Maryasin, B.; Marquetand, P.; Maulide, N. Machine Learning for Organic Synthesis: Are Robots Replacing Chemists? Angew. Chemie Int. Ed. 2018, 57 (24), 6978–6980. https://doi.org/https://doi.org/10.1002/anie.201803562. |
| [7] | Zhou, Z.; Li, X.; Zare, R. N. Optimizing Chemical Reactions with Deep Reinforcement Learning. ACS Cent. Sci. 2017, 3 (12), 1337–1344. https://doi.org/10.1021/acscentsci.7b00492. |
| [8] | Wei, J. N.; Duvenaud, D.; Aspuru-Guzik, A. Neural Networks for the Prediction of Organic Chemistry Reactions. ACS Cent. Sci. 2016, 2 (10), 725–732. https://doi.org/10.1021/acscentsci.6b00219. |
| [9] | Head, M. L.; Holman, L.; Lanfear, R.; Kahn, A. T.; Jennions, M. D. The Extent and Consequences of P-Hacking in Science. PLOS Biol. 2015, 13 (3), e1002106. |
| [10] | Kerr, N. L. HARKing: Hypothesizing After the Results Are Known. Personal. Soc. Psychol. Rev. 1998, 2 (3), 196–217. https://doi.org/10.1207/s15327957pspr0203_4. |
| [11] | Faber, J.; Fonseca, L. M. How Sample Size Influences Research Outcomes. Dental Press J. Orthod. 2014, 19 (4), 27–29. https://doi.org/10.1590/2176-9451.19.4.027-029.ebo. |
| [12] | Button, K. Reboot Undergraduate Courses for Reproducibility. Nature 2018, 561 (7723), 287. https://doi.org/10.1038/d41586-018-06692-8. |
| [13] | Chanda, A.; Daly, A. M.; Foley, D. A.; LaPack, M. A.; Mukherjee, S.; Orr, J. D.; Reid, G. L.; Thompson, D. R.; Ward, H. W. Industry Perspectives on Process Analytical Technology: Tools and Applications in API Development. Org. Process Res. Dev. 2015, 19 (1), 63–83. https://doi.org/10.1021/op400358b. |
| [14] | Carter, C. F.; Lange, H.; Ley, S. V; Baxendale, I. R.; Wittkamp, B.; Goode, J. G.; Gaunt, N. L. ReactIR Flow Cell: A New Analytical Tool for Continuous Flow Chemical Processing. Org. Process Res. Dev. 2010, 14 (2), 393–404. https://doi.org/10.1021/op900305v. |
| [15] | Foley, D. A.; Doecke, C. W.; Buser, J. Y.; Merritt, J. M.; Murphy, L.; Kissane, M.; Collins, S. G.; Maguire, A. R.; Kaerner, A. ReactNMR and ReactIR as Reaction Monitoring and Mechanistic Elucidation Tools: The NCS Mediated Cascade Reaction of α-Thioamides to α-Thio-β-Chloroacrylamides. J. Org. Chem. 2011, 76 (23), 9630–9640. https://doi.org/10.1021/jo201212p. |
| [16] | Rueping, M.; Bootwicha, T.; Sugiono, E. Continuous-Flow Catalytic Asymmetric Hydrogenations: Reaction Optimization Using FTIR Inline Analysis. Beilstein J. Org. Chem. 2012, 8, 300–307. |
| [17] | Bertram, A.; Davies, E. S.; Denton, R.; Fray, M. J.; Galloway, K. W.; George, M. W.; Reid, K. L.; Thomas, N. R.; Wright, R. R. From Cook to Chef: Facilitating the Transition from Recipe-Driven to Open-Ended Research-Based Undergraduate Chemistry Lab Activities. New Dir. Teach. Phys. Sci. No 10 2014. |
| [18] | Barnes, L.; Blaber, H.; Brooks, D. T. K.; Byers, L.; Buckley, D.; Byron, Z. C.; Chilvers, R. G.; Cochrane, L.; Cooney, E.; Damian, H. A.; Francis, L.; Fu He, D.; Grace, J. M. J.; Green, H. J.; Hogarth, E. J. P.; Jusu, L.; Killalea, C. E.; King, O.; Lambert, J.; Lee, Z. J.; Lima, N. S.; Long, C. L.; Mackinnon, M.-L.; Mahdy, S.; Matthews-Wright, J.; Millward, M. J.; Meehan, M. F.; Merrett, C.; Morrison, L.; Parke, H. R. I.; Payne, C.; Payne, L.; Pike, C.; Seal, A.; Senior, A. J.; Smith, K. M.; Stanelyte, K.; Stillibrand, J.; Szpara, R.; Taday, F. F. H.; Threadgould, A. M.; Trainor, R. J.; Waters, J.; Williams, O.; Wong, C. K. W.; Wood, K.; Barton, N.; Gruszka, A.; Henley, Z.; Rowedder, J. E.; Cookson, R.; Jones, K. L.; Nadin, A.; Smith, I. E.; Macdonald, S. J. F.; Nortcliffe, A. Free–Wilson Analysis of Comprehensive Data on Phosphoinositide-3-Kinase (PI3K) Inhibitors Reveals Importance of N-Methylation for PI3Kδ Activity. J. Med. Chem. 2019, 62 (22), 10402–10422. |
| [19] | Bailie, A. E.; Nortcliffe, A. Synthesis of Quinolone Antibiotic Analogues: A Multistep Synthetic Chemistry Experiment for Undergraduates. J. Chem. Educ. 2021, 98 (10), 3333–3340. https://doi.org/10.1021/acs.jchemed.1c00459. |
| [20] | Iwai, Y. Modified McFadyen-Stevens Reaction for a Versatile Synthesis of Aromatic Aldehydes. Org. Synth. 2018, 95, 276–288. https://doi.org/10.15227/orgsyn.095.0276. |
| [21] | Reid, N.; Shah, I. The Role of Laboratory Work in University Chemistry. Chem. Educ. Res. Pract. 2007, 8 (2), 172–185. https://doi.org/10.1039/B5RP90026C. |
| [22] | Davies, J. C.; Pattison, D.; Hirst, J. D. Machine learning for yield prediction for chemical reactions using in situ sensors. J. Mol. Graph. Model. 2023, 118, 108356. https://doi.org/10.1016/j.jmgm.2022.108356. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML