-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2022; 10(2): 21-24
doi:10.5923/j.jlce.20221002.01
Received: May 23, 2022; Accepted: Jun. 15, 2022; Published: Jun. 23, 2022

Green Chemistry Based Synthesis of Silver Nanoparticles from Eggshells to Develop Electrochemical Sensor for Common Neurotransmitter Detection
Riley Rush, Ryan Shuler, Suzanne K. Lunsford
Department of Chemistry, Wright State University, Dayton, USA
Correspondence to: Suzanne K. Lunsford, Department of Chemistry, Wright State University, Dayton, USA.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This lab experiment involved the modified silver nanoparticles synthesized for the detection of catechol in the presence of ascorbic acid in acidic and physiological pH conditions. The modified green synthesized silver nanoparticles (AgNP) working carbon electrode showed enhanced detection of catechol compared to the bare electrode utilizing Differential Pulse Voltammetry (DPV). These silver nanoparticles synthesized by green chemistry (household chemicals) offer novel and potential alternative to chemically toxic solvent synthesized nanoparticles. The various alternative household chemicals to synthesize the AgNPs will be compared using eggshell, honey and orange peel to illustrate the optimized synthesis in the development of the modified carbon sensor electrode to detect catechol and ascorbic acid simultaneously by DPV in acidic conditions. The eggshell synthesized AgNP electrode were studied to detect lower concentrations of catechol at pH of 7.4, and found to respond better than acidic conditions plus more selective to catechol than ascorbic acid at physiological pH of 7.4. Additionally, the UV-Vis data confirms the successful synthesis of the AgNPs that were deposited onto the working electrode, peak on UV-Vis formed around 450 nm. This novel experiment could be utilized to train undergraduate students on green chemistry synthesis integrated with electrochemistry techniques such as differential pulse voltammetry.
Keywords: Electrochemistry, Green Chemistry, Silver Nanoparticles, Electrochemical Sensors
Cite this paper: Riley Rush, Ryan Shuler, Suzanne K. Lunsford, Green Chemistry Based Synthesis of Silver Nanoparticles from Eggshells to Develop Electrochemical Sensor for Common Neurotransmitter Detection, Journal of Laboratory Chemical Education, Vol. 10 No. 2, 2022, pp. 21-24. doi: 10.5923/j.jlce.20221002.01.
Article Outline
1. Introduction
- There have been substantial efforts in the development of electrochemical sensors based on electrodes modified to enhance the electrocatalytic ability to detect neurotransmitters such as catechol. Therefore, the need for a reliable detection method of catechol has been sought after over three decades. The ability to detect catechol in the human body is important in the determination of brain activity. Catechol has a 1,2-dihydroxybenzene structure where the redox reaction takes place similar to common neurotransmitters such as dopamine, and epinephrine. The structure for the catechol reaction is:
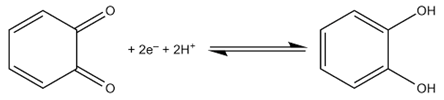 The frequency of these oxidation/reduction reactions can help determine the brain activity of a person, with abnormal reaction rates hinting at some sort of mental illness, such as Parkinson's. The issue with detecting catechol in the body is that other chemicals undergo redox reactions, such as ascorbic acid (AA) [1-2]. These chemicals are very common in the human body and can easily interfere with the detection of neurotransmitters. The detection of common neurotransmitters such as catechol electrochemically in the presence of common interferences such as ascorbic acid could be optimized by enhancing the electrocatalytic activity of the working carbon electrode surface sensor. The morphology of the working electrode sensor was optimized by synthesizing silver nanoparticles to be deposited on to working electrode surface. Due to our limited availability of instrumentation such as an scanning electron microscope or transmission electron microscopy thus in our experiment the best instrumentation available was the UV-Vis spectra to confirm the synthesized AgNPs. Using these metal nanoparticles may seem like an expensive method of catechol detection, but the synthesis of the nanoparticles can be achieved with common household items, including honey, orange peels, and eggshells. Using household materials to synthesize nanoparticles without the use of more toxic and dangerous chemicals prevents production of more hazardous waste. These green alternatives for silver nanoparticle synthesis were utilized to modify the carbon working electrode to detect catechol in the presence of a common interference (ascorbic acid) with Differential Pulse Voltammetry (DPV). DPV was chosen as an electrochemical technique due to selectivity, sensitive, dependable and cost-effective [3]. This project has been focused on synthesizing the silver nanoparticles (AgNPs) onto a working electrode that will be studied for sensitivity, selectivity and reproducibility of neurotransmitter detection which will be a novel experiment to integrate nanoparticle sensors and electrochemistry. Spectroscopic techniques such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) can be utilized to monitor neurotransmitters such as catechol. However techniques such as PET which are noninvasive have low spatial and temporal resolution plus the high cost of PET and fMRI need to be considered. Electrochemistry techniques such as DPV require no background subtraction and are highly selective for neurotransmitters without high cost compared to PET and fMRI instrumentation. In vivo detection of neurotransmitters in the presence of common interferences allows for real time measurements that are not extremely expensive with quick results utilizing electrochemistry techniques such as cyclic voltammetry and differential pulse voltammetry.
The frequency of these oxidation/reduction reactions can help determine the brain activity of a person, with abnormal reaction rates hinting at some sort of mental illness, such as Parkinson's. The issue with detecting catechol in the body is that other chemicals undergo redox reactions, such as ascorbic acid (AA) [1-2]. These chemicals are very common in the human body and can easily interfere with the detection of neurotransmitters. The detection of common neurotransmitters such as catechol electrochemically in the presence of common interferences such as ascorbic acid could be optimized by enhancing the electrocatalytic activity of the working carbon electrode surface sensor. The morphology of the working electrode sensor was optimized by synthesizing silver nanoparticles to be deposited on to working electrode surface. Due to our limited availability of instrumentation such as an scanning electron microscope or transmission electron microscopy thus in our experiment the best instrumentation available was the UV-Vis spectra to confirm the synthesized AgNPs. Using these metal nanoparticles may seem like an expensive method of catechol detection, but the synthesis of the nanoparticles can be achieved with common household items, including honey, orange peels, and eggshells. Using household materials to synthesize nanoparticles without the use of more toxic and dangerous chemicals prevents production of more hazardous waste. These green alternatives for silver nanoparticle synthesis were utilized to modify the carbon working electrode to detect catechol in the presence of a common interference (ascorbic acid) with Differential Pulse Voltammetry (DPV). DPV was chosen as an electrochemical technique due to selectivity, sensitive, dependable and cost-effective [3]. This project has been focused on synthesizing the silver nanoparticles (AgNPs) onto a working electrode that will be studied for sensitivity, selectivity and reproducibility of neurotransmitter detection which will be a novel experiment to integrate nanoparticle sensors and electrochemistry. Spectroscopic techniques such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) can be utilized to monitor neurotransmitters such as catechol. However techniques such as PET which are noninvasive have low spatial and temporal resolution plus the high cost of PET and fMRI need to be considered. Electrochemistry techniques such as DPV require no background subtraction and are highly selective for neurotransmitters without high cost compared to PET and fMRI instrumentation. In vivo detection of neurotransmitters in the presence of common interferences allows for real time measurements that are not extremely expensive with quick results utilizing electrochemistry techniques such as cyclic voltammetry and differential pulse voltammetry. 2. Experimental
2.1. Laboratory Equipment
- A three-electrode single compartment cell was utilized for the voltammetry studies with the platinum foil as the auxiliary, and Ag/AgCl/3 M NaCl (MF-2074, BAS) as the reference, and a polished carbon electrode (1.6 mm) with the green synthesized Ag nanoparticles was deposited onto the working electrode. Bioanalytical Systems Epsilon potentiostat-galvanostat instrument was utilized to carry out the DVP data.
2.2. Nanoparticle Synthesis
- The Ag nanoparticles were synthesized by dissolving various household extracts in water. For the honey AgNPs, 20 grams of honey was dissolved in 80 mL of water at room temperature. The eggshell extract was made by dissolving 10 grams of crushed eggshell in about 450 mL of near boiling water for 30 minutes. In the case of the orange peel extract, 10 grams of the peel was dissolved in near boiling water for 30 minutes. 20 mL of silver nitrate and 15 mL of the resulting solution were mixed in a vial and left in a dark area for about four days. In this time, the AgNPs would form naturally from the interaction of the silver nitrate and the polyphenol structures found in the extract used. The UV-Vis below in Figure 1., confirms the successful synthesis a nanoparticle with a AgNP peak around 450 nm [4-7].
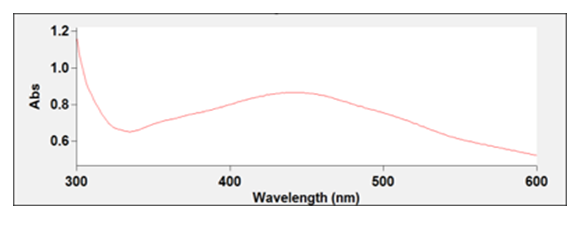 | Figure 1. UV-Vis of silver nanoparticles synthesized by eggshells |
3. Results and Discussion
3.1. Electrode Modification
- A carbon electrode was polished and coated with the AgNPS via dropper. The AgNPs were left to dry in the open air until the liquid is mostly gone. It is important to note some of the major contamination issues can occur in running tests by DPV with electrodes coated in AgNPs if glassware and electrodes are not cleaned appropriately. When making the solutions for testing the coated electrodes, it is important to make sure that all of the glassware involved is clean. If any glassware has a small amount of contamination, including the testing cell and the storing container for the chemicals, the reading will be affected by the contaminant. If the working electrode is not properly cleaned, the reading will also be changed. It is vital to dry the AgNPs on the working electrode, and if not thoroughly dried, can result in a weak signal and a poor reading overall.
3.2. Comparison of Honey AgNPs, Eggshell AgNPs, and Orange Peel AgNPs as a Detection Method
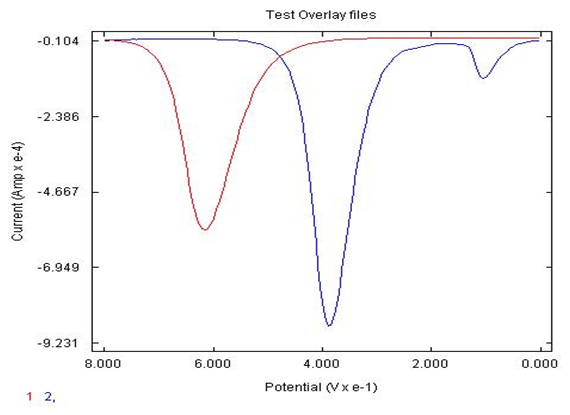
- The synthesis of AgNPs were compared by different household items which were honey, eggshell and orange peels. Upon investigation, it was found that the honey, eggshell and orange peel has ascorbic acid in it naturally, so using these AgNPs would always result in the showing the AgNPs have a slight signal showing the detection of ascorbic acid as well (100 mV-approximate signal). In order to determine what would give the optimized response to catechol, the three samples were compared as shown in Figure 2. In comparing the three sets of AgNPs, it was found that the eggshell AgNPs were better overall in detecting catechol with a more enhanced signal. Note the eggshell signal for catechol is approximately 2 times greater than the signal for honey in detection of catechol, (peak around 400 mV).
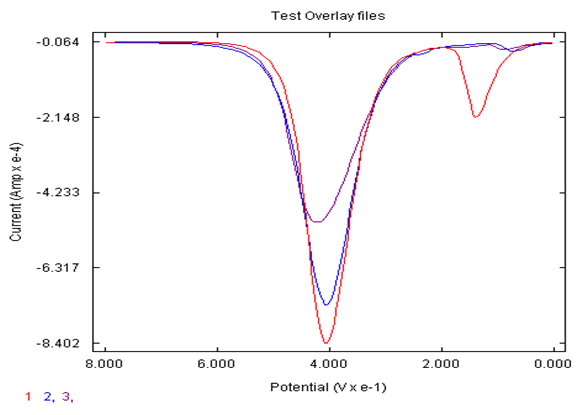 | Figure 2. DPV of AgNPs made from 1) eggshell, 2) orange peel, and 3)honey deposited on carbon electrode in detection of 0.05 M catechol in 0.1 M sulfuric acid |
3.3. Comparison of Eggshell AgNPs, and Bare Carbon to Detect Catechol and AA Simultaneously by DPV
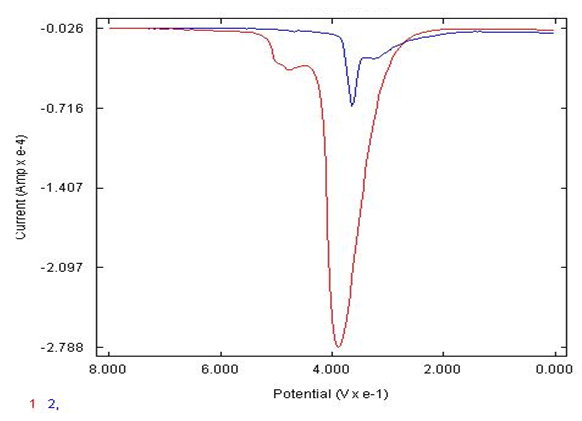
- The detection of ascorbic acid in the presence of catechol has been an interference issues for over 5 decades in electrochemical techniques such as cyclic voltammetry, square wave voltammetry and differential pulse voltammetry due to similar potential at which these analytes oxidize. The bare carbon electrode response in the detection of catechol in the presence of ascorbic acid as shown in Figure 3 has no peak detection for catechol. However, the AgNPs synthesized by eggshell on the modified carbon electrode show the mixture of catechol and ascorbic acid successfully detected where two peaks are signified on the DPV. The bare carbon electrode cannot detect the ascorbic acid peak and there is a poor signal for catechol as well compared to the modified eggshell AgNP electrode. The bare carbon electrode only signified one peak thus cannot detect catechol and ascorbic acid simultaneously thus interference issues compared to the modified AgNP electrode synthesized nanoparticles by eggshell signified two peaks thus successful detection of ascorbic acid and catechol simultaneously.
3.4. Comparison of Eggshell AgNPs, and Bare Carbon to Detect Catechol and AA at Different pH Values Acidic Versus Physiological pH Values of 7.4 by DPV
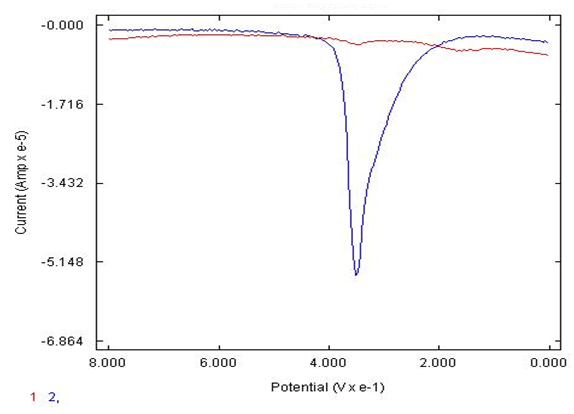
- Figure 4 shows that at the lower concentration of 5X10-5 M catechol in phosphate buffer, pH of 7.4, was detected well with the eggshell AgNP carbon electrode compared to the bare carbon electrode with significantly poorer response. The synthesized AgNP (eggshell) electrode was found to have an enhanced signal at physiological pH values of 7.4 to detect 5X10-5 M catechol on phosphate buffer compared to pH values of 2, acidic conditions for 5X10-5 M catechol in sulfuric acid as noted in Figure 5. The physiological pH is the most important aspect that the sensor can work in the real world range to detect the neurotransmitters such as catechol in the presence of common interferences. Figure 6., illustrates that the modified AgNP sensor can successfully detect the catechol at pH of 7.4 in the presence of AA with low concentrations and more selective for the catechol. In the physiological pH 7.4, there are resolution issues that need to be considered, as shown in Figure 6 (one peak of catechol in the presence of AA). However, two peaks for mixture of catechol and AA, figure 3. This confirms that pH is a factor in the response of the AgNP electrode. Therefore, acidic conditions with the eggshell AgNP electrode, as depicted in Figure 3, there are two distinguishable peaks, the catechol peak and AA peak, (100 mV AA peak and at 400 mV is the catechol peak in sulfuric acid, pH of 2). The bare carbon electrode is not successful at more basic pH range to detect the 10-5 M catechol in the presence of ascorbic acid thus no real response signal for the neurotransmitter of interest.
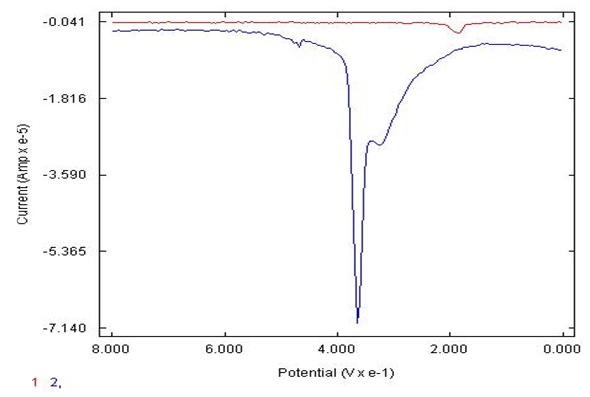 | Figure 4. DPV of bare carbon electrode (1) versus eggshell AgNP carbon electrode (2) to detect 5X10-5 M catechol in 0.1 M phosphate buffer, pH of 7.4 |
4. Conclusions
- The green chemistry proved to be an environment friendly and effective approach for the synthesis of nanoparticles thus remains to be an alternative method to the chemical reduction method. The rapid biosynthesis of the silver nanoparticles are extremely fast, inexpensive, ecofriendly and stable for several weeks. The eggshells are generally discarded into the environment, so synthesis of nanoparticles and then employing them in certain allocation is a novel idea [7]. This study has demonstrated that the eggshell AgNP modified electrodes exhibited very interesting electrocatalytic properties which can be utilized in the detection of catechol in the presence of common interferences such as ascorbic acid. Electrode modification with AgNPs made from eggshells provided better selectivity in the DPV measurements of the catechol in the presence of ascorbic acid. A linear regression (R2) of 0.9209 was found when the current versus concentration were plotted for the DPV data in physiological pH conditions to detect catechol. Overall good linear regression response for an undergraduate students data learning novel techniques. Although realization of these selectivity and sensitivity enhancements in neurological studies would require miniaturization of the AgNP working electrode and further studies would be required to examine more interferences such as uric acid for real world sensor production. However, this low cost and simple synthesis by green chemistry technology seems to be a great utility for further development due to the successful response of the modified AgNP carbon electrode sensor to detect low concentrations of catechol at physiological pH of 7.4, using DPV [3]. Our students have found this experiment rewarding and has allowed the expansion of content knowledge in green chemistry integrated with electrochemistry techniques to optimize electrochemical sensors morphology for real-world analysis of neurotransmitters such as catechol.
ACKNOWLEDGEMENTS
- This work has been supported by the National Science Foundation, ASK (Applying Scientific Knowledge) program -grant no. 1742339. The authors would like to thank the National Science Foundation and Wright State University.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML