-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2022; 10(1): 9-19
doi:10.5923/j.jlce.20221001.03
Received: May 11, 2022; Accepted: May 25, 2022; Published: Jun. 13, 2022

Using Imidazolium-Based Ionic Liquids to Teach Fundamental NMR Interpretation
Neil L. Heckman, Morgan M. Wohl, Jordyn D. Duffy
Department Chemistry and Physics, Hastings College, Hastings, NE, USA
Correspondence to: Neil L. Heckman, Department Chemistry and Physics, Hastings College, Hastings, NE, USA.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This experiment is designed for an undergraduate organic chemistry course when students are first introduced to NMR concepts. It also introduces students to the topic of ionic liquids, which is often ignored in many undergraduate organic chemistry courses. This simple and straightforward synthesis of four unique molecules provides samples that can be analyzed using 1H NMR and 13C NMR with teaching grade instrumentation. These examples can help students to understand the fundamental concepts of chemical shift values and multiplicity numbers with compounds simple to synthesize.
Keywords: Ionic Liquids, Imidazolium, NMR, Organic Chemistry
Cite this paper: Neil L. Heckman, Morgan M. Wohl, Jordyn D. Duffy, Using Imidazolium-Based Ionic Liquids to Teach Fundamental NMR Interpretation, Journal of Laboratory Chemical Education, Vol. 10 No. 1, 2022, pp. 9-19. doi: 10.5923/j.jlce.20221001.03.
Article Outline
1. Introduction
- Ionic liquids have been studied extensively in recent years, yet they are often times ignored in many undergraduate chemistry courses and textbooks [1-2]. They have a wide variety of uses, but most are thought of as catalysts that can easily be recycled in green chemistry and in the use in batteries [3]. Synthesis of smaller imidazolium-based ionic liquid is fairly straight forward and can be done in a variety of solvents without the aid of nitrogen. This simple method has been shown to be an effective tool in the organic chemistry undergraduate laboratory [4]. Although some research has been documented recently using teaching grade NMR instrumentation, this is not the case with many papers. Compounds are often too complex for students to analyze using a teaching grade NMR and can often be confusing to students beginning to learn this concept [5]. With the greater availability of many different imidazole reagents, several small imidazolium ionic liquids can be synthesized with unique 1NMR characteristics that can be used to help students learn the fundamentals of this technique using a teaching grade, low MHz NMR. Proton NMR is a common skill taught in most undergraduate organic chemistry courses. Many students find this skill initially difficult to learn, especially the interpretation of chemical shift values and multiplicity numbers and can benefit from multiple approaches [6]. This paper documents a straight forward laboratory exercise to synthesize several imidazolium-based ionic liquids having unique 1H NMR characterizes that can aid in learning this information, while demonstrating a synthesis of a potentially useful class of compounds that have not received much emphasis in the undergraduate study of organic chemistry.
2. Experimental
- Four different imidazolium-based ionic liquids were synthesized that produced low melting point solids that are stable under atmospheric conditions. The four imidazole compounds used were 1-methylimidazole, 1-ethylimidazole, 1-isopropylimidazole, and 1-tert-butylimidazole. The two alkyl halides used were methyl iodide and ethyl iodide. All reagent chemicals were commercially available from multiple vendors. All procedures were conducted under a chemical fume hood. Appropriate PPE and other safety precautions were taken using the laboratory standards, with all waste disposed of using the appropriate local guidelines and regulations.
2.1. Synthetic Procedure
- A solution of 1-alkylimidazole (20 mmol), 25 mL of methanol, and a slight excess of alkylhalide (25 mmol) was mixed and allowed to reflux for 24 hrs using a reflux apparatus with a drying tube attached (figure 1). After the reflux was complete, the solvent and any unreacted alkyl halide was removed using rotary evaporation to yield a low melting point solid. The crude product was washed with 10 mL of tert-butyl methyl ether twice to remove any unreacted imidazole and decanted. Any residual ether was removed using rotary evaporation and samples were analyzed using melting point, 1H NMR, and 13C NMR. These procedures were modified from a previous published study [7]. Since 1-ethyl-3-methylimidazolium iodide is hydroscopic, samples were capped placed in a desiccator prior to analysis. Alternatively, this sample can be analyzed immediately after the sample is removed from the final rotary evaporation.
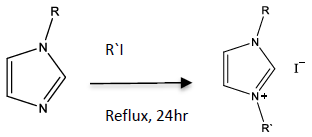 | Figure 1. Synthetic scheme for the formation of a dialkylimidazolum iodide |
2.2. Compound Characterization
- Samples were analyzed using a 60 MHz Anasazi NMR. Melting point was determined using a Mel-Temp II melting point apparatus and the values were uncorrected.
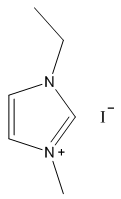
2.2.1. 1-Ethyl-3-Methylimidazolium Iodide
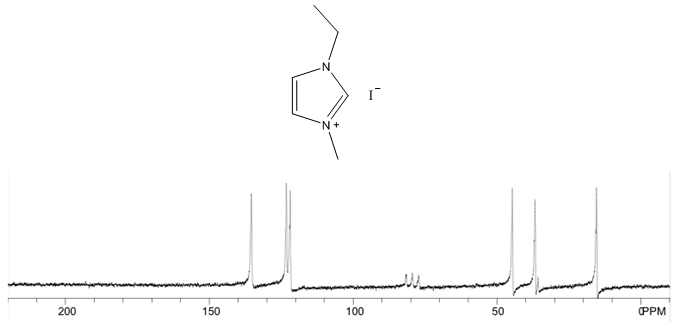
- Yellow solid, m.p. 56-70°C, 60% yield, 1H NMR (CDCl3) δ: 10.05 (s, 1H), 7.53 (dd, 2H), 4.47 (q, 2H), 4.12 (s, 3H), 1.63 (t, 3H) 13C NMR (CDCl3) δ: 135.4, 123.2, 121.9, 44.7, 36.8, 15.2.
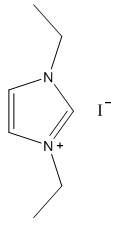
2.2.2. 1,3-Diethylimidazolium Iodide
- White solid, m.p. 110-114°C, 92% yield, 1H NMR (CDCl3) δ: 9.89 (s, 1H), 7.86 (dd, 2H), 4.53 (q, 4H), 1.65 (t, 6H) 13C NMR (CDCl3) δ: 134.7, 122.0, 44.8, 15.4.
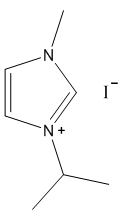
2.2.3. 1-Isopropyl-3-Methylimidazolium Iodide
- White solid, m.p. 94-97°C, 94% yield, 1H NMR (CDCl3) δ: 10.03 (s, 1H), 7.63 (dd, 2H), 4.5-4.8 (m, 1H), 4.15 (s, 3H), 1.60 (d, 6H) 13C NMR (CDCl3) δ: 135.8, 124.3, 121.2, 53.8, 37.6, 23.9.
2.2.4. 1-tert-Butyl-3-Methylimidazolium Iodide
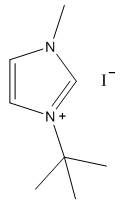
- White solid, m.p. 125-127°C, 94% yield, 1H NMR (CDCl3) δ: 10.02 (s, 1H), 7.66 (dd, 2H), 4.19 (s, 3H), 1.75 (s, 9H) 13C NMR (CDCl3) δ: 136.1, 125.1, 121.0, 61.6, 38.1, 31.2.
3. Discussion
- The four compounds synthesized have distinct characteristics, especially with the 1H NMR spectra that can be used to help students understand this concept. Although the purity may not be research quality in some cases, the samples are certainly pure enough to show all representative peaks and demonstrate the fundamental concepts in an undergraduate course. All of the samples contain an imidazolium structure with 3 hydrogens attached to the aromatic ring. These hydrogens have chemical shift values greater than 7 ppm with two of the peaks close to each other (they may even show up as one single peak with an integration of 2H) and one singlet peak distinctly higher that corresponds to the hydrogen that is adjacent to both nitrogens. Next, all of the samples have at least one branch attached to the imidazolium with a carbon containing hydrogens, directly attached to a nitrogen. These peaks have chemical shift values between 4-5 ppm that should not be confused with the hydrogens that are part of the imidazolium or the hydrogens in the branches that are further away from the imidazolium. Methyl branches exhibit a multiplicity of a singlet with an integration of 3H, while ethyls show as quartets with an integration of 2H, and isopropyls as a multiplet with an integration of 1H. Tert-butyl branches do not have a carbon attached to the imidazolium with hydrogens and the absence of a peak should be noted. The hydrogens further away from the imidazolium all have chemical shift values less than 2 ppm and should not be confused with the two other types of peaks described earlier. The tert-butyl branch has a singlet peak with an integration of 9H corresponding to the 3 symmetric CH3 branches. Isopropyl branches have a doublet with an integration of 6H corresponding to the two symmetric CH3 branches, while ethyl branches have a triplet with an integration of 3H corresponding to the CH3 adjacent to a CH2. The 13C NMR is useful to show that the three carbons associated with the imidazolium have values between 120 and 140 ppm with the two carbons adjacent to each other having similar values, while the carbon adjacent to both nitrogens has a distinctly higher value. The peaks associated with the symmetric carbons in tert-butyl have a size approximately three times larger than the other alkyl carbon peaks and the peaks associated with the two symmetric carbons in the isopropyl branch have a peak approximately twice as large as the other alkyl carbon peaks. It is also easy to see the peak corresponding to each unique carbon in the molecules. The peaks for both 1H NMR and 13C NMR were similar to what was predicted using ChemDraw [8].Visual observation and melting point can also be used to help in the identification of these molecules, although it is not the main focus of this experiment. Each sample is a low melting point solid. All samples are white or off white, with the 1-ethyl-3-methylimidazolium iodide being a yellow solid. The melting points of each sample is also quite unique with a wide melting point range expected for 1-ethyl-3-methylimidazolium iodide as it most likely absorbed some water, even with the precautions taken. The nature of ionic liquids may result in higher than usual variability in melting point ranges for students.
4. Conclusions
- This simple SN2 reaction is robust and should be suitable for an undergraduate organic chemistry laboratory. The NMR spectra were made using a 60 MHz 1H NMR (15 MHz 13C NMR) with adequate resolution and could be adapted to a benchtop NMR. These molecules can serve as great examples when introducing NMR concepts, while exposing students to the topic of ionic liquids.
Supporting Information
- 1-ethyl-3-methylimidazolium iodide – 13C NMR Spectrum
 1-ethyl-3-methylimidazolium iodide – 13C NMR Spectrum Predicted from ChemDraw
1-ethyl-3-methylimidazolium iodide – 13C NMR Spectrum Predicted from ChemDraw 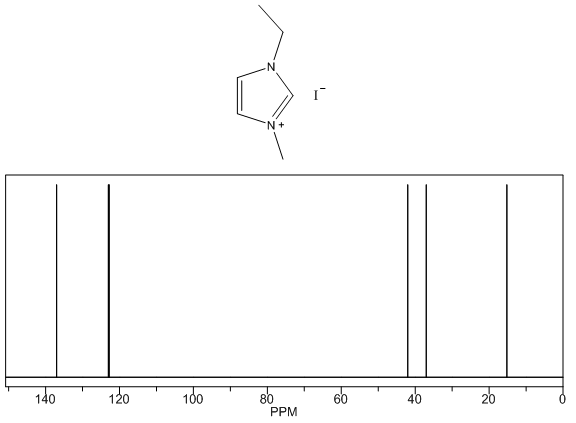 1-ethyl-3-methylimidazolium iodide – 1H NMR Spectrum
1-ethyl-3-methylimidazolium iodide – 1H NMR Spectrum 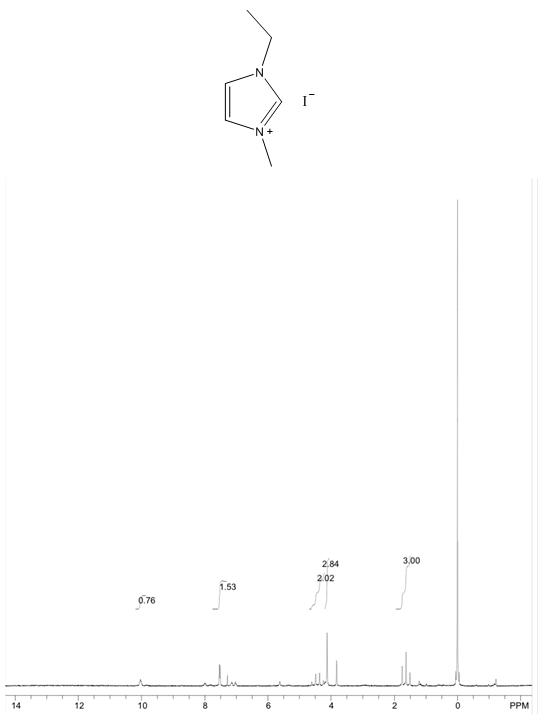 1-ethyl-3-methylimidazolium iodide – 1H NMR Spectrum Predicted from ChemDraw
1-ethyl-3-methylimidazolium iodide – 1H NMR Spectrum Predicted from ChemDraw 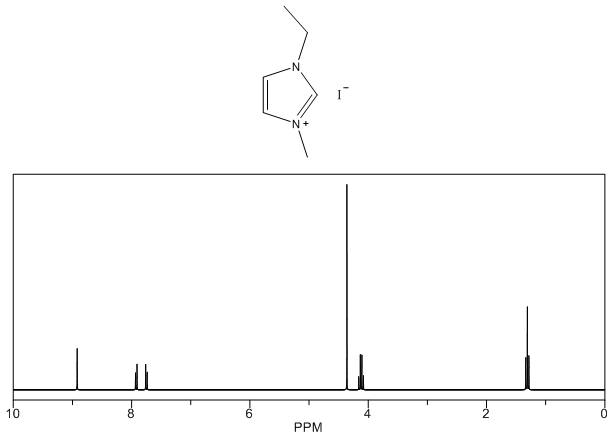 1,3-diethylimidazolium iodide – 13C NMR Spectrum
1,3-diethylimidazolium iodide – 13C NMR Spectrum 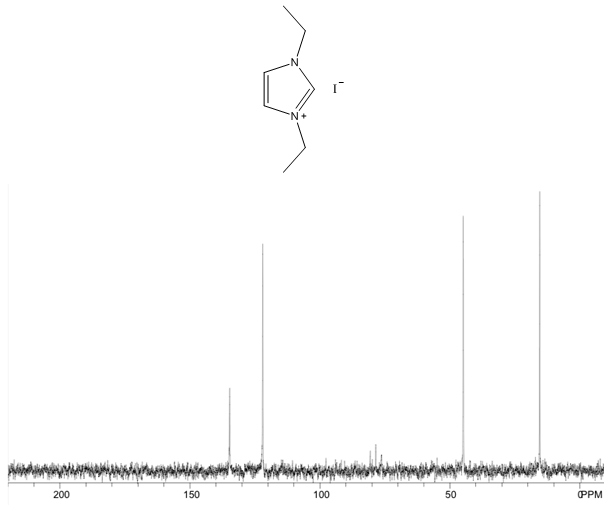 1,3-diethylimidazolium iodide – 13C NMR Spectrum Predicted from ChemDraw (only one resonance structure)
1,3-diethylimidazolium iodide – 13C NMR Spectrum Predicted from ChemDraw (only one resonance structure)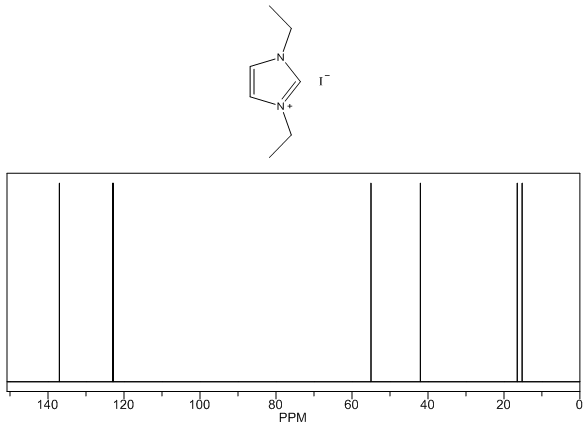 1,3-diethylimidazolium iodide – 1H NMR Spectrum
1,3-diethylimidazolium iodide – 1H NMR Spectrum 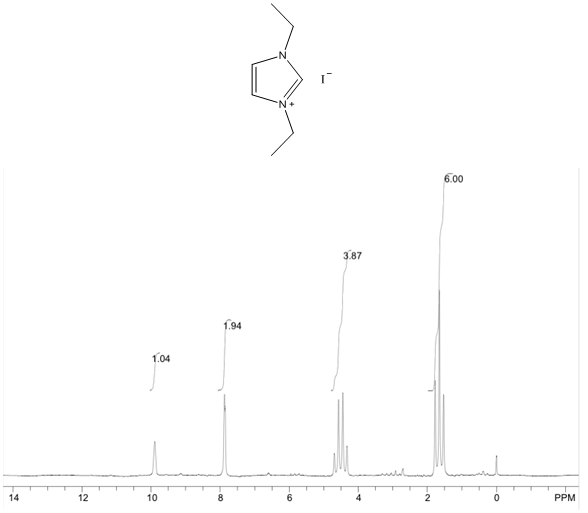 1,3-diethylimidazolium iodide – 1H NMR Spectrum Predicted from ChemDraw (only one resonance structure)
1,3-diethylimidazolium iodide – 1H NMR Spectrum Predicted from ChemDraw (only one resonance structure)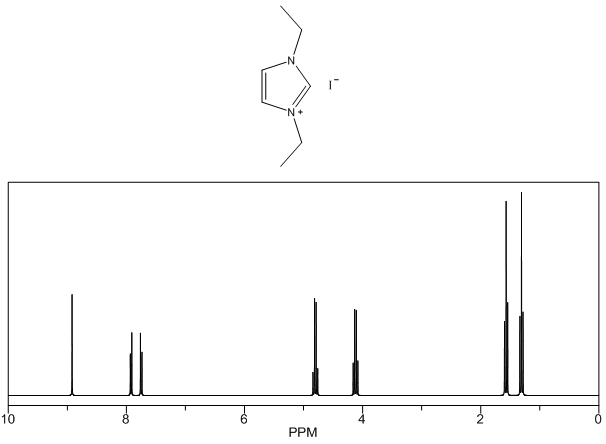 1-isopropyl-3-methylimidazolium iodide – 13C NMR Spectrum
1-isopropyl-3-methylimidazolium iodide – 13C NMR Spectrum 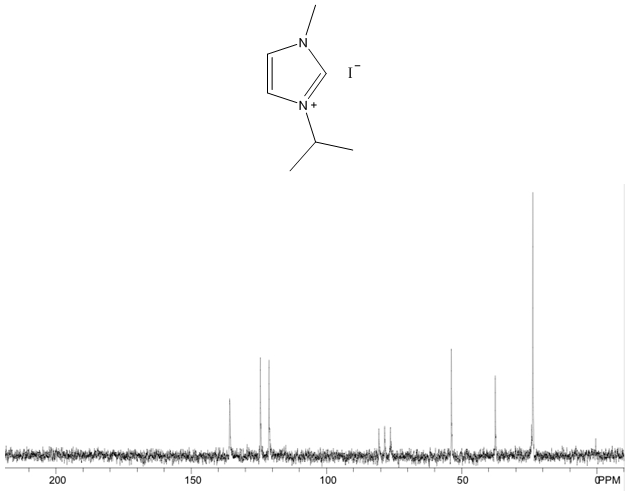 1-isopropyl-3-methylimidazolium iodide – 13C NMR Spectrum Predicted from ChemDraw
1-isopropyl-3-methylimidazolium iodide – 13C NMR Spectrum Predicted from ChemDraw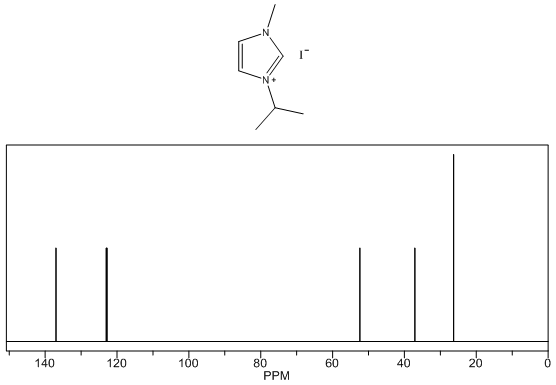 1-isopropyl-3-methylimidazolium iodide – 1H NMR Spectrum
1-isopropyl-3-methylimidazolium iodide – 1H NMR Spectrum 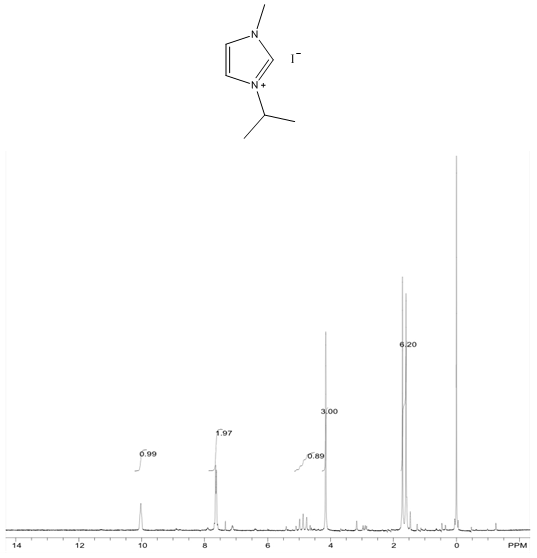 1-isopropyl-3-methylimidazolium iodide – 1H NMR Spectrum Predicted from ChemDraw
1-isopropyl-3-methylimidazolium iodide – 1H NMR Spectrum Predicted from ChemDraw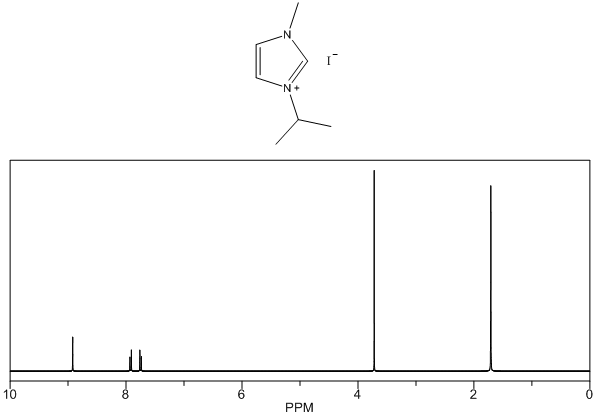 1-tert-butyl-3-methylimidazolium iodide – 13C NMR Spectrum
1-tert-butyl-3-methylimidazolium iodide – 13C NMR Spectrum 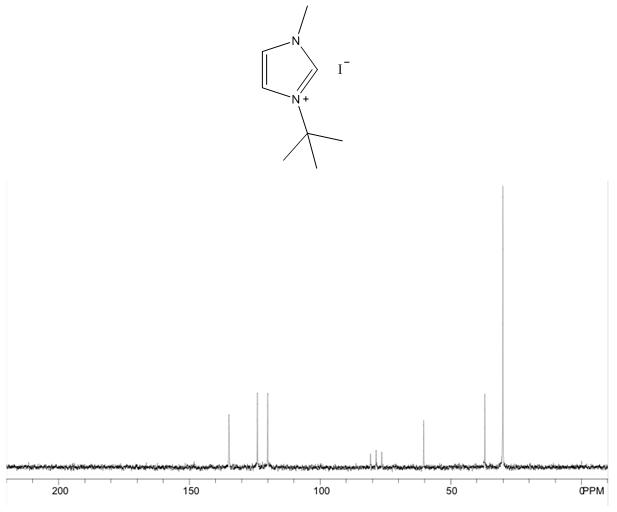 1-tert-butyl-3-methylimidazolium iodide – 13C NMR Spectrum Predicted from ChemDraw
1-tert-butyl-3-methylimidazolium iodide – 13C NMR Spectrum Predicted from ChemDraw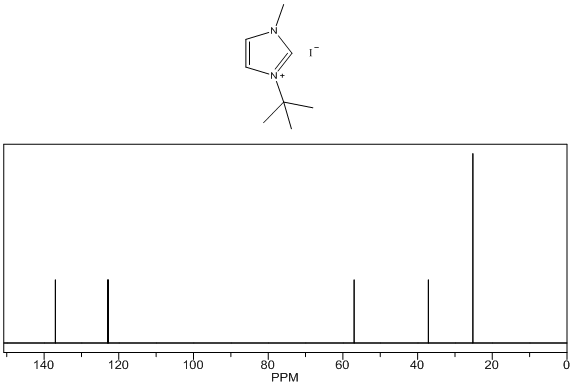 1-tert-butyl-3-methylimidazolium iodide – 1H NMR Spectrum
1-tert-butyl-3-methylimidazolium iodide – 1H NMR Spectrum 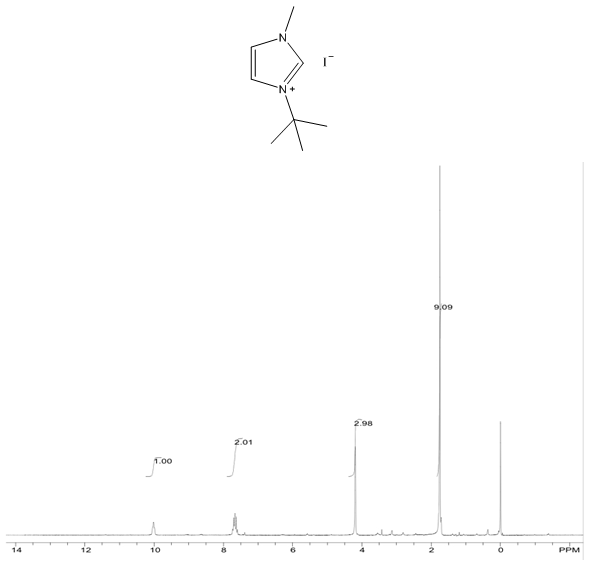 1-tert-butyl-3-methylimidazolium iodide – 1H NMR Spectrum Predicted from ChemDraw
1-tert-butyl-3-methylimidazolium iodide – 1H NMR Spectrum Predicted from ChemDraw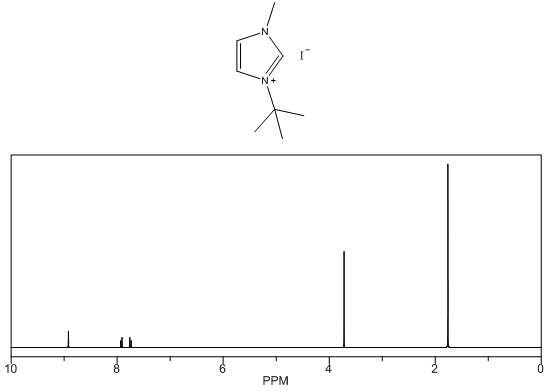
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML