-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2022; 10(1): 4-8
doi:10.5923/j.jlce.20221001.02
Received: Mar. 10, 2022; Accepted: Mar. 25, 2022; Published: Apr. 15, 2022

A Glass Vessel Insulating Cup GVIC Calorimeter to Study Chemical Reactions in the Liquid Phase
Tarek R. Farhat, Hasan El Rifai, Rana Jisr
Physical Sciences, West Virginia University Institute of Technology, Beckley, WV, USA
Correspondence to: Tarek R. Farhat, Physical Sciences, West Virginia University Institute of Technology, Beckley, WV, USA.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

An alternative setup to the classical cup calorimetry to monitor simple chemical reactions in the liquid phase is introduced and discussed. One of the most common calorimetry procedures used in college chemistry labs is the foam or coffee cup reactor. In this paper, we will test and evaluate a Glass Vessel Insulating Cup (GVIC) Calorimeter composed of parts readily available in labs but inherently different in design from a regular cup calorimeter. Results obtained were well within the statistical limit and the GVIC technique saved more than 5 times on solution consumption. The reaction system studied is the enthalpy of neutralization using a dilute alkaline solution of NaOH and acidic solutions of either HCl or H2SO4 solutions.
Keywords: Aqueous Solution Chemistry, Calorimetry, Thermochemistry, Neutralization, Enthalpy
Cite this paper: Tarek R. Farhat, Hasan El Rifai, Rana Jisr, A Glass Vessel Insulating Cup GVIC Calorimeter to Study Chemical Reactions in the Liquid Phase, Journal of Laboratory Chemical Education, Vol. 10 No. 1, 2022, pp. 4-8. doi: 10.5923/j.jlce.20221001.02.
Article Outline
1. Introduction
- Extensive Thermodynamic properties like enthalpy change ∆H and entropy change ∆S of a reaction are studied in academic labs using simple apparatus. Other thermodynamic quantities such as Gibbs free energy change ∆G and equilibrium constant Kc of a reaction are obtained from ∆H and entropy change ∆S [1,2,3]. Most popular calorimetry/thermochemistry experiments are dissolution, neutralization, and dilution reactions [1,2,3]. In most experiments heat is either liberated or absorbed and the rise or drop in temperature is monitored using a calorimeter setup. Any Temperature increase is indicative of an exothermic process which in general associates with –∆H or –∆G. Students usually perform experiments to measure the heat capacity of the cup calorimeter, Cc, otherwise, it will be provided as an approximate measure [1,2]. A simple and cheap cup-calorimeter setup conducted at high schools and undergrad/general chemistry levels would use foam cups, polystyrene cups, or paper coffee cups as the core of the experiment [1,2,4,5,6]. The objective of the published setups is to seek low cost and easy to assemble chemistry experiments for chemical education [7,8,9,10]. In most cases cups come without a cover, thus, cardboard lids with a hole are used instead. A hole in the lid is made to accommodate the thermometer. Care must be taken not to puncture the foam or paper cup when stirring or fitting the thermometer. To avoid using the approximate value of the heat capacity, Cc, of the cup-calorimeter, students can spend half the lab’s time working to get the temperature profile of the hot-cold water mixture [2], though the use of electronic equipment reduced it to a few minutes [11]. To achieve a certain level of acceptable accuracy, a cup-Calorimeter would need at least 50 mL of each liquid to run the experiment. That is, the cup reactor can easily accommodate a 100 mL mixture. A typical class setting of 20 students would need at least 1.0 liter of acid solution and 1 liter of the base solution. Normally, two acids (e.g. HCl, H2SO4) are used, making the volume required up to 2 liters each. A prudent Lab supervisor would prepare double the quantity to account for solution losses and repetition of experimental procedures which requires 2L of acid solution HCl, 2L of acid solution H2SO4, and 4L of NaOH solution. A problem arises at universities, colleges, and high schools of multiple sections, where volumes can easily exceed 15 liters for general chemistry lab prep work to conduct experiments such as neutralization reactions. Handling such large volumes is inconvenient to lab managers and supervisors.In this paper, we propose using easy to assemble Calorimeter that we call a “Glass Vessel Insulating Cup” (GVIC) Calorimeter where temperature can be accurately monitored while solution volume consumption is cut off by four to five times. Note, although a foam cup is used it is not the reactor. The purpose of the foam cup or any cup is to add another layer of insulation to the glass vessel which is the reactor. Liquids are poured into the glass vessel and not the foam cup. With a glass vessel, most solutions or liquids commonly used in educational laboratories can be poured inside the glassy reactor to study different types of chemical reactions in the liquid phase. For example, the GVIC can study reactions of liquids that would otherwise dissolve the foam cup. Thus, widens the versatility of the proposed method to organic liquids rather than aqueous liquids only. The GVIC heat capacity is accurately determined by weighing the glass vessel so no time is wasted on finding, Cc, of a calorimeter. Our CHEM115L students used the GVIC to determine the enthalpy of neutralization reaction:
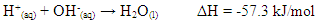 In most experiments conducted by our students, temperature changes of the reaction mixture exceeded 5°C and were accurately measured to determine enthalpy changes.
In most experiments conducted by our students, temperature changes of the reaction mixture exceeded 5°C and were accurately measured to determine enthalpy changes.2. Experimental Procedure
- Under this section, we will discuss hardware and chemicals, logistics, the heat capacity of the calorimeter, calorimeter setup, and hazards.
2.1. Chemicals and Hardware
- Chemicals were used from the supplier without any further processing. Reagent NaOH 99% pearls from Alfa-Aesar, HCl acid 37%, and sulfuric acid 98% from Sigma. Solutions of 2.0 M HCl, 2.0M NaOH, and 1.0 M H2SO4 were prepared using DI water, analytical balance, grad cylinders, and volumetric flasks. Acid solutions were stored in glass bottles while NaOH solutions were in plastic bottles ready for students’ use. Foam cups, cotton plugs, glass bottles (1 oz), digital thermometers, and aluminum foil are part of the normal equipment in our laboratories and were used as-is.
2.2. Logistics and Procedure
- The “Glass Vessel Insulating Cup” (GVIC) Calorimeter experiment was performed by two different groups. The first group comprised teaching assistants at West Virginia University Institute of Technology (WVU-Tech) who obtained and verified results independent from students taking the CHEM115L course. The 2nd group comprised undergrad students of the CHEM115L course at WVU-Tech registered in various sections and semesters.
2.3. Heat Capacity of the Calorimeter
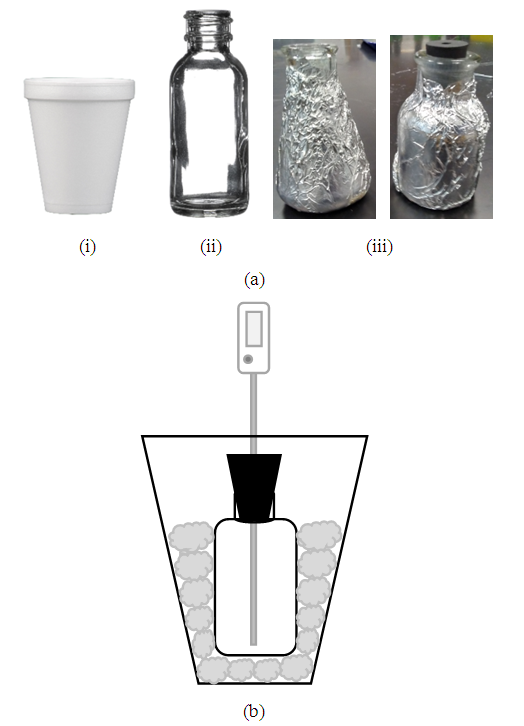
- In the first part of the experiment, the glass vessel is either a 30 mL glass bottle (1oz bottle) or 25 mL Erlenmeyer flask, Figure-1 (a-i, a-ii). The glass bottle (~ 3 mm wall thickness) was weighed either using a top-loading balance of a tolerance ±0.01 g or an analytical balance with 100 g capacity. The mass of the glass bottle, Mgl, can be used later to determine the heat capacity of the calorimeter, Ccal. Next, is to prepare the calorimeter setup.
2.4. Calorimeter Setup to Monitor Temperature Change
- (i) Parts needed: Cotton plugs, foam cup, and aluminum foil for insulation and support. Two glass bottles with a one-hole rubber stopper. A digital thermometer to monitor temperature change of the neutralization reaction. (ii) Procedure: Cotton plugs are placed at the bottom of the foam cup to act as a cushion. The glass bottle or flask must be covered with aluminum foil wrapped around it in two layers, Figure-1 (a-iii).
2.5. Hazards
- Hydrochloric acid HCl, sulfuric acid H2SO4, and sodium hydroxide NaOH reagent bottles contain corrosive chemicals and should be handled with care. A prudent measure is to prepare respective dilute solutions of all reagents using the fume hood, gloves, aprons, and goggles. Always pour concentrated liquids of acids over pure water in volumetric flasks. After reaching the dilution mark in a volumetric flask, freshly prepared acid solutions tend to shrink in volume and overheat. Keep checking on the mark and add more pure water when the level drops below it. With gloves on, homogenize the mixture after each addition of pure water. All solutions of NaOH should be stored in PP or PE bottles. Dilute solutions of 2.0 M NaOH, 2.0 M HCl, and 1.0 M H2SO4 can be used on Lab benches to conduct enthalpy change experiments using full gear of PPE.
3. Results and Discussion
- In Part I of this experiment, students would determine the calorimeter constant of the “Glass Vessel Insulating Cup (GVIC)” calorimeter”. To a first approximation, the surrounding of the neutralization reaction in aqueous media is confined to the glass container only. The foam cup, rubber stopper, and cotton insulation are not included because these are not in direct contact with the liquid and their contribution to heat capacity is negligible. The mass of insulating aluminum foil is in the range of 1.5 to 2.5 g with a specific heat capacity, sAl, of 0.9 Jg-1°C-1. The specific heat capacity of soft glass, sgl, is 0.84 Jg-1°C-1 and the mass range of the glass containers (i.e. glass bottles or jars, flasks,.. etc.) is between 55.0 g to 65.0 g. If the mass of aluminum foil is ignored it will introduce an error of 3.5%±0.5%. Instructors are welcome to add the contribution of the insulation material mentioned per their lab setup. After the mass of the glass bottle, Mgl, is measured students were able to calculate the approximate heat capacity of Calorimeter, Ccal, such that,
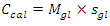 | (1) |
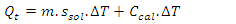 | (2) |
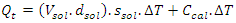 | (3) |
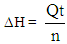 | (4) |
3.1. Method Validation
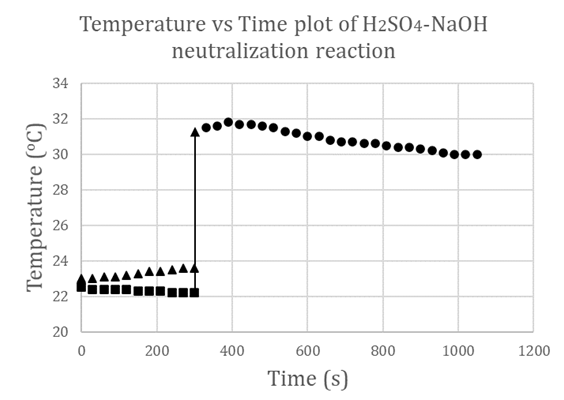
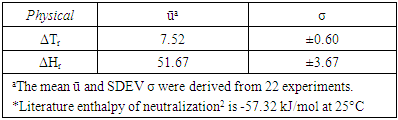
- Using equations (1) to (4), we can calculate values of heat gained by solution mixture, Qmix, and the heat gained by calorimeter, QCal, thus be able to obtain the total heat of the reaction, Qt, in J. The enthalpy of neutralization ΔH can be determined experimentally per the amount of acid or base used. To validate the proposed experimental method, we used two sources of data. The first source, experiments were carefully conducted and monitored for reproducibility by teaching assistants where their results were used as a reference, while the second source is the data gathered from CHEM115L students’ electronic reports. We managed to gather a population of twenty-two independent data sets. For the enthalpy of neutralization experiment, the two parameters that were considered for statistical analysis are the temperature rise ΔT (°C) and the enthalpy change ΔH (kJ/mol). The mean ū and the standard deviation σ of ΔTs and ΔHs of these reports were calculated. From the first source, experiments were carefully conducted to minimize errors, so these were considered our standard reference frame. The reference temperature change determined was ΔTr = 7.4°C and the corresponding enthalpy change is ΔHr = 51.67 kJ/mol. From the second source, where data is expected to scatter and fluctuate according to students’ performance, the mean ū and the standard deviation σ are calculated using a spreadsheet and conveniently presented in Table 1.
|
3.2. The t-test
- For a normal distribution of ΔTs values, a reasonable range of temperature change was obtained between 4 to 11°C. In terms of %population, ~68% of the data points fall within ū ± 1.σ and ~95% fall within ū ± 2.σ range. The confidence interval CI of a t-test where t = 2.08 at 95% confidence level and n -1 = 21 degrees of freedom is determined from,
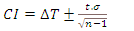 | (5) |
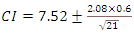 | (6) |
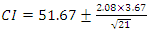 | (7) |
4. Conclusions
- In this paper, we have introduced a technical modification to the conventional foam or coffee cup calorimeter, that is the “Glass Vessel Insulating Cup” (GVIC) calorimeter to study simple laboratory chemical reactions. The proposed experimental technique uses insulated small glass vessels thus offering many advantages. The GVIC (i) shortens the time of the experiment, (ii) makes ~5 times saving on solutions expenditure, (iii)hence less waste to dispose of, (iv) allows a greater variety of chemicals and solutions to react in a glass reactor where polystyrene, foam, or paper cup fails, (v) uses apparatus that is simple to assemble in any educational lab.
ACKNOWLEDGEMENTS
- The authors would like to thank all students of West Virginia University Institute of Technology who contributed to this work directly or indirectly.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML