-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2022; 10(1): 1-3
doi:10.5923/j.jlce.20221001.01
Received: Feb. 13, 2022; Accepted: Feb. 28, 2022; Published: Mar. 15, 2022

Spot Test to Differentiate between D-Glucose and D-Fructose
Sharda Mahilkar Sonkar, Sujata Sengupta, Shivani Singh
Department of Chemistry, Miranda House, University of Delhi, Delhi
Correspondence to: Sujata Sengupta, Department of Chemistry, Miranda House, University of Delhi, Delhi.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Qualitative analysis of an unknown organic compound is an important component of the chemistry curriculum in most universities of India. Students at both the undergraduate and post graduate level along with students majoring in biochemistry and life sciences are required to perform the qualitative analysis of organic compounds as part of their coursework. The analysis of carbohydrates using the well-known Molisch’s test is routinely performed. However, distinguishing between D-Glucose and D-Fructose using the known standard is usually a challenging task. The present work highlights a simple spot test to differentiate between D-Glucose and D-Fructose by modification of the existing Molisch’s test, based on the principles of green chemistry.
Keywords: Qualitative Organic Analysis, Carbohydrates, D-Glucose, D-Fructose, Molisch’s test
Cite this paper: Sharda Mahilkar Sonkar, Sujata Sengupta, Shivani Singh, Spot Test to Differentiate between D-Glucose and D-Fructose, Journal of Laboratory Chemical Education, Vol. 10 No. 1, 2022, pp. 1-3. doi: 10.5923/j.jlce.20221001.01.
1. Introduction
- Determination of the functional group of an unknown organic compound by using the standard classification tests is an essential part of the undergraduate chemistry curriculum in most universities. [1] Identification of carbohydrates is an important aspect of qualitative organic analysis. A positive Molisch test, indicated by the formation of violet ring, detects the presence of carbohydrates and distinguishes them from other kinds of organic substances. [2] Furthermore, carbohydrates are characterised as being reducing or non-reducing depending on their structure. A positive test with Tollens', Fehling's, and Benedict's test reagents are indicative of the presence of reducing sugars. [2,3]In the teaching laboratory, both D-glucose and D-fructose are two common carbohydrates routinely provided to the students for analysis. While both compounds do well in all of the aforementioned tests, however, students commonly struggle to distinguish between the two during identification, when given as an unknown. Both D-glucose and D-fructose have a wide melting point range, hence, the common practice of using melting point to determine the identity of either of the two is not useful. [3] Moreover, both D-glucose and D-fructose form the same osazone derivative (glucosazone, needle-shaped crystals). [4] Preparation of other suitable characterisation derivatives (e.g., acetates) necessitate extensive experimental skills which are not typically performed by the undergraduate students.In recent years, the use of spot tests for functional group analysis on grooved tiles have been reported and has gained much popularity as it affords an efficient, safe and economical approach. [5] In continuation with our institutions continuing efforts in adopting safer and “greener” practices in the undergraduate laboratory [6], herein we report a simple spot test to distinguishes between D-glucose and D-fructose by modification of the standard Molisch’s Test. Furthermore, the spot test is performed using only 1-2 drops of the reagents and often added with the help of a capillary. Therefore, the spot test provides an economical, less wasteful and safer approach to the analysis.
2. Reagents Required
- Saturated solution of D-Glucose and D-Fructose10% alcoholic solution of α-naphtholConcentrated Sulphuric Acid solution (25 N)Procedure: In a clean, dry groove spotting tile, two drops of the saturated sugar solution is added. A drop of alcoholic solution of α-naphthol is added to the respective slot, followed by the immediate addition of 1-2 drops of conc. H2SO4. D-Fructose shows the formation of a violet colour within 15-20 s while D-Glucose shows an easily distinguishable pink colour after 50-55 s. (Figure 1)
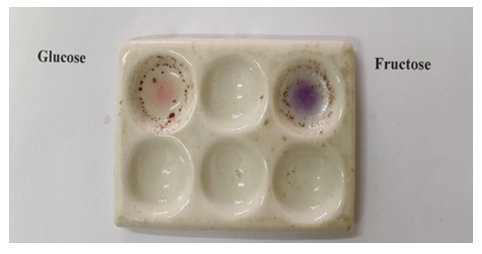 | Figure 1. Spot test to distinguish between D-glucose and D-fructose performed on a groove spotting tile |
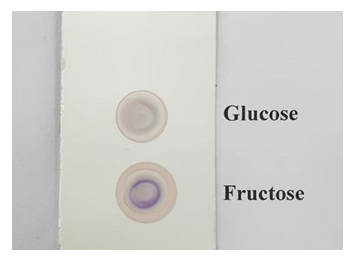 | Figure 2. Spot test to distinguish between D-glucose and D-fructose performed on a Merck TLC Silica plate |
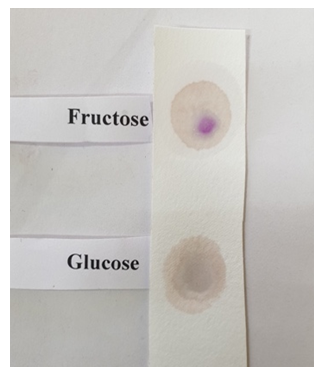 | Figure 3. Spot test to distinguish between D-glucose and D-fructose performed on a Whatman™ Filter Paper (ashless, Grade 42) |
3. Discussion
- The Molisch’s test is a common test for the qualitative analysis of carbohydrates. [7] The test is based on the fact that pentoses and hexoses are readily dehydrated by conc. sulphuric acid to form a furfural or hydroxy methyl furfural derivatives, which condense with α-naphthol to form a purple condensation product, visible as a violet ring at the junction formed between the H2SO4, the sugar solution and Molisch’s reagent mixture. [7-8] The results from the above modified Molisch’s spot tests indicate that D-fructose reacts faster than D-glucose. This is probably due to a quicker formation of the furfural derivative on dehydration with conc. sulphuric acid as compared D-glucose. [9] Hence, the two carbohydrates may be easily distinguished from each other. For the spot tests, the reagents used in the traditional macroscale Molisch’s analysis have been used. All the reagents are commonly available in any undergraduate chemistry laboratory. Furthermore, the amounts of the individual reagents used is significantly reduced, thereby, diminishing the risk and hazards associated with the use of concentrated sulphuric acid. Less waste is generated and reactions are clean, avoiding the use of typical apparatus such as test tubes and droppers. Finally, the spot test analysis scheme produces reliable and repeatable results. The current research is therefore a step toward educating young and aspiring scientists about adopting green methods in the organic laboratory.
ACKNOWLEDGEMENTS
- The present work has been carried out under the D. S. Kothari Centre for Research and Innovation in Science Education, Miranda House, University of Delhi. We are thankful to The Principal, Miranda House, for the permission and encouragement to pursue the study. We would also like to thank Dr Sunita Dhingra for her continued guidance for the work. We are thankful to our undergraduate students and laboratory staff who provided help.
Conflict of Interest
- There is no conflict of interest amongst the authors.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML