-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2021; 9(3): 41-49
doi:10.5923/j.jlce.20210903.02
Received: Aug. 24, 2021; Accepted: Sep. 30, 2021; Published: Oct. 22, 2021

Distance Teaching of the Undergraduate Laboratory During Pandemic Time: Dissolution of Cellulose in Mixtures of Ionic Liquids and Dimethyl Sulfoxide and Biopolymer Regeneration as Films
Nicolas Keppeler, Matheus Costa Lourenço, Omar A. El Seoud
Institute of Chemistry, the University of São Paulo, São Paulo, SP, Brazil
Correspondence to: Matheus Costa Lourenço, Omar A. El Seoud, Institute of Chemistry, the University of São Paulo, São Paulo, SP, Brazil.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

We report on distance teaching of an experimental project, taught during an advanced chemistry course given to our chemistry-major students (47, divided into eight groups). The project required 12 h to complete, during three weeks. We chose a subject of socio-economic relevance, namely, the physical dissolution of cellulose (Cel), an important step in processing wood-based Cel to fabricate, e.g., fibers and films. The solvents employed were mixtures of DMSO with (green) ionic liquids (ILs) 1-allyl-3-methylimidazolium acetate- (AlMeImAcO) and chloride (AlMeImCl). The former IL dissolves more Cel than the latter, this difference in efficiency was probed using molecular dynamics (MD) simulations. The dissolved biopolymer was dyed with a reactive dye and regenerated as films. The online activities during the classes included showing videos of the experiments done by the instructor; explaining how MD simulations of Cel dissolution were done, and seminars given by the students on themes related to the project. The seminars were on the commercial production of Viscose and Lyocell Cel fibers, dyeing of cellulosic fibers, and properties (light- and washing fastness) of dyed textiles. Additionally, the students were asked to suggest experiments to corroborate, or refute the results of MD simulations. The students appreciated our active learning approach (85.1%); reported that they learned new material and found the seminar themes interesting (80.9%). They indicated that online classes do not replace face-to-face practical classes (59.6%), and that the activities given do not replace the experience acquired by doing the experiment (66.0%). Regarding their local environment, 74.5% considered the conditions at home as good/very good (internet connection and hardware). After returning to face-to-face teaching, we will continue showing videos of the staff while carrying out the experiments, before going to the laboratory. This is a very useful approach to highlight important experimental precautions/protocols e.g., regarding the proper handling of chemicals and equipment. We recommend this project (distance-, or face-to-face teaching) for students of science courses because of its relevance, safety, and low-cost.
Keywords: Online teaching, Practical courses, Green solvents, Ionic Liquids, Cellulose Dissolution, Cellulose Film Regeneration, Molecular Dynamics Simulations
Cite this paper: Nicolas Keppeler, Matheus Costa Lourenço, Omar A. El Seoud, Distance Teaching of the Undergraduate Laboratory During Pandemic Time: Dissolution of Cellulose in Mixtures of Ionic Liquids and Dimethyl Sulfoxide and Biopolymer Regeneration as Films, Journal of Laboratory Chemical Education, Vol. 9 No. 3, 2021, pp. 41-49. doi: 10.5923/j.jlce.20210903.02.
Article Outline
1. Introduction
- Note: A list of Abbreviations and Acronyms is given after Acknowledgements.In response to the SARS-CoV2 pandemic in 2020, health authorities imposed restrictions (e.g., social distancing and wearing facial masks) to curb the spread out of the virus. Consequently, most schools and universities worldwide shifted to online teaching, an alternative that has been employed for some time before the pandemic [1]. However, using the same approach to teach experimental courses is a challenging task, because the students do not do the experiments. We should take this aspect into account when teaching online practical courses, especially because the above-mentioned restrictions will most probably be in effect in many countries through 2021, due to the painfully slow, and unequal pace of vaccination [2].We report here on teaching an online project as a part of an advanced experimental chemistry course (4 hours/week) given to our chemistry-major students during the April to July semester of 2021. The number of enrolled students was 47, these were divided into 8 groups (each of five or 6 students); the activities reported here required 12 h. In conformance with the education for sustainable development approach (ESD) declared by the UNESCO [3], we chose a subject of socio-economic relevance, namely, the physical dissolution of cellulose, a reaction that occurs without formation of covalent bonds. This is an important step in the processing of cellulose (Cel) extracted from wood and other lignocellulosic starting materials to obtain, e.g., fibers, films, nanoparticles [4]. Cel dissolution is also a promising recycling strategy for post-fabrication rejects (from the production of garments), and that of post-use (old clothes) [5]. The solvent we employed to dissolve Cel is a mixture of dimethyl sulfoxide (DMSO) and, as green solvents, the ionic liquids (ILs) 1-allyl-3-methylimidazolium acetate (AlMeImAcO) and chloride (AlMeImCl). We synthesized the latter using microwave (MW) heating as unconventional energy source [6].The online activities during the classes included showing videos of the experiments done by the instructor, explaining how molecular dynamics (MD) simulations of Cel dissolution were done, and seminars given by the students on themes related to the experiments. Additionally, the students were asked to suggest an experiment to corroborate or refute the results of MD simulations that indicated the following order of Cel dissolution efficiency: AlMeImAcO > AlMeImCl.
2. Experimental
2.1. Reagents and Cellulose Samples
- The reagents/solvents (name, CAS number): 3-chloro-1-propene (allyl chloride, 107-05-1), cellobiose (528-50-7), deuterated chloroform (865-49-6; 99.8 atom% D), 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU, 6674-22-2), DMSO (67-68-5), commercial absolute ethanol (64-17-5), ethyl acetate (141-78-6), 1-methylimidazole (616-47-7), potassium chloride (7447-40-7), and Reactive Blue 2 dye (12236-82-7) were purchased from Acros Organics and Synth (São Paulo), and were employed as received. The Cel samples included microcrystalline cellulose (MCC; Avicel PH101; 9004-34-6) purchased from FMC, and cotton powder (9004-34-6) supplied by Dr. Daniela C. Ferreira of the Institute for Technological Research (São Paulo).
2.2. Equipment
- We used CEM Discover model DU-8316 MW oven for IL synthesis, and Bruker Avance 300 spectrometer (300 MHz for 1H) for recording the 1H NMR spectra (CDCl3 solvent with TMS reference). We used a PC-controlled Metrohm model 827 pH-meter/ion meter equipped with Metrohm 6.0910.120 conductivity micro-electrode (4 mm diameter) to record solution conductivity, via a RS-232 serial port. We carried out this experiment using a double-walled conductivity cell through which water is circulated from a thermostat [7].
2.3. Syntheses of the Ionic Liquids
- The syntheses of AlMeImCl and AlMeImAcO were carried out as given elsewhere, and are schematically depicted in Scheme 1. We reacted 1-methylimidazole (20 mL; 0.25 mol) and (cold) allyl chloride (19.6 mL; 0.24 mol) in 75 mL of ethyl acetate, first at room temperature (30 min), and then under MW irradiation (100 W, reflux, 2 hours). After cooling, the lower layer (IL) was separated and washed with 20 mL of cold ethyl acetate. The latter was separated and agitated with water (vortex mixer); the pH of the aqueous phase was measured. After three such treatments, the water was neutral. This IL was also the starting material for the synthesis of AlMeImAcO. The ion exchange (AlMeImCl → AlMeImAcO) was done on a microporous ion exchange resin (Amberlite IRN 78, 1.20 equiv. OH− L-1), using methanol as eluent, as given elsewhere for the synthesis of the IL 1-butyl-3-methylimidazolium acetate, BuMeImAcO [8]. Both ILs were dried under reduced pressure at 60°C; they showed the expected 1H NMR spectra [9].
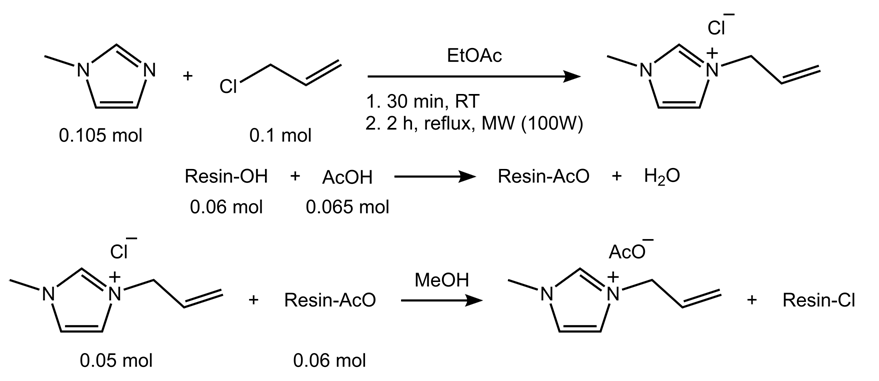 | Scheme 1. Scheme for the synthesis of 1-allyl-3-methylimidazolium chloride, AlMeImCl and 1-allyl-3-methylimidazolium acetate, AlMeImAcO |
2.4. Cellulose Dissolution in Mixtures of DMSO and the Ionic Liquids
- We carried out these experiments using the same protocol employed for Cel dissolution in BuMeImAcO-DMSO mixture, using an in-house constructed dissolution equipment, see Figure 1 [8].
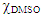 = 0.6), dissolution temperature = 70°C; agitation speed, 150 rpm; MCC concentration = 10.0 wt%. The biopolymer dissolution was followed visually, and under a microscope using plane-polarized light (Nikon model Eclipse E200). Under these experimental conditions MCC dissolution was incomplete for AlMeImCl (max. = 5.3 wt%), and complete for AlMeImAcO, see Figure 2.
= 0.6), dissolution temperature = 70°C; agitation speed, 150 rpm; MCC concentration = 10.0 wt%. The biopolymer dissolution was followed visually, and under a microscope using plane-polarized light (Nikon model Eclipse E200). Under these experimental conditions MCC dissolution was incomplete for AlMeImCl (max. = 5.3 wt%), and complete for AlMeImAcO, see Figure 2.2.5. Dyeing of Dissolved Cotton Cellulose and Regeneration of Cellulose Films
- For the formation of cellulose films, we used cotton powder because it gives films with better mechanical properties. After dissolution of 4 wt% cotton powder in 15 g of AlMeImAcO-DMSO (
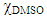 = 0.6; at 70°C; 30 min) we added 5 drops of the superbase DBU (to ionize the dissolved Cel) and ca. 1 mg of Reactive Blue 2. The still warm solution was then stirred with a vortex mixer for 30 minutes and regenerated as a semi-transparent blue Cel film as follows: we poured the warm solution onto a 2.5 x 7.5 cm glass plate and spread the solution over the surface with a spatula. We waited several minutes to allow the surface to become homogeneous (see Figure 3A), placed the glass plate into a Petri dish with ethanol and washed it carefully until the film started to separate from the glass plate. Then, we carefully removed the film, placed it again in the Petri dish and washed it with ethanol for 15 minutes (the ethanol was changed twice), and dried the film with a heat gun, see Figure 3B and C.
= 0.6; at 70°C; 30 min) we added 5 drops of the superbase DBU (to ionize the dissolved Cel) and ca. 1 mg of Reactive Blue 2. The still warm solution was then stirred with a vortex mixer for 30 minutes and regenerated as a semi-transparent blue Cel film as follows: we poured the warm solution onto a 2.5 x 7.5 cm glass plate and spread the solution over the surface with a spatula. We waited several minutes to allow the surface to become homogeneous (see Figure 3A), placed the glass plate into a Petri dish with ethanol and washed it carefully until the film started to separate from the glass plate. Then, we carefully removed the film, placed it again in the Petri dish and washed it with ethanol for 15 minutes (the ethanol was changed twice), and dried the film with a heat gun, see Figure 3B and C.2.6. Molecular Dynamics Simulation of the Dissolution of Cellulose in Mixtures of DMSO with AlMeImCl and AlMeImAcO
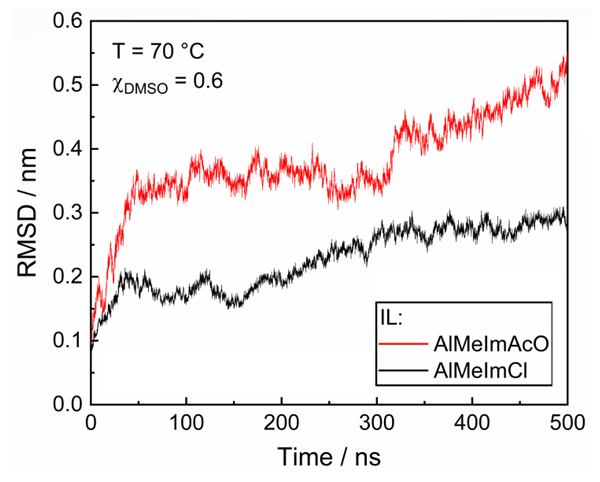
- We used Gromacs 2020 software package [11] to simulate these systems, each containing the following number of molecules: DMSO, 1500; IL, 1000; and a model for cellulose crystallite. The latter was composed of an ensemble of 8 chains of (β-1,4-linked) anhydroglucose units (AGUs), each one has 10 AGUs (hereafter called ensemble). We built the ensemble with Cellulose-Builder script [12] and generated the simulation boxes using the PACKMOL program [13].We performed the simulation at 343 K (70°C), for 500 ns by using OPLS (Optimized Potential for Liquid Simulations) force field for all molecules, isothermal-isobaric (NPT) condition, periodic boundaries, and the smooth particle-mesh Ewald (PME) algorithm for long-range electrostatic interactions [14]. We checked the equilibration of the ensemble by monitoring the potential energy as a function of simulation time. We found that the latter curves typically reached equilibrium values (i.e., remains essentially constant) after ca. 50 ns. We optimized the geometries (gas phase) of the IL cations and the acetate ion using DFT calculations, with B3LYP functional and 6-31G(d,p) basis set, as implemented in Gaussian 09 [15]. We generated the topology files of OPLS force field using the MKTOP [16] and calculated the partial charges on the atoms using the RESP (restrained electrostatic potential fit) approach [17] as calculated by Antechamber 12 tools [18]. We used published data for OPLS-optimized DMSO geometry and topology [19]. We analyzed the MD simulation results using root mean square deviations (RMSD) tools, implemented in the Gromacs package.
2.7. Dependence of the Conductivity of Cellobiose Solutions in Ionic Liquid-DMSO Mixtures on the Disaccharide Concentration
- We calibrated the micro-electrode with 0.01 mol L-1 KCl solution at 25°C as recommended elsewhere [20]. We added 3 g of the IL-DMSO binary mixture (
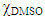 = 0.6) to the conductivity cell, sealed the inlets of the latter with silicone rubber septa and kept at 40°C. We drilled a hole into the central septum and inserted the conductivity electrode. This setup avoids solution contamination with adventitious moisture. Additionally, care should be taken to eliminate any air bubbles from the micro-electrode cavity. We performed the “titration” of the IL-DMSO by adding 0.05 g aliquots of dried cellobiose to the solution and recorded the conductivity after complete dissolution of the cellobiose, which required ca. 20 minutes after each addition of the disaccharide.
= 0.6) to the conductivity cell, sealed the inlets of the latter with silicone rubber septa and kept at 40°C. We drilled a hole into the central septum and inserted the conductivity electrode. This setup avoids solution contamination with adventitious moisture. Additionally, care should be taken to eliminate any air bubbles from the micro-electrode cavity. We performed the “titration” of the IL-DMSO by adding 0.05 g aliquots of dried cellobiose to the solution and recorded the conductivity after complete dissolution of the cellobiose, which required ca. 20 minutes after each addition of the disaccharide. 2.8. Hazards
- The IL synthesis part of this project (carried out by the instructor/students) should be done in a fume hood with efficient ventilation. The operator should use personal protective equipment (safety goggles, gloves, etc.). Allyl chloride is a flammable liquid, harmful by skin absorption, irritant, and carcinogen; 1-methylimidazole can cause skin burns and eye damage. Ethyl acetate is flammable and should be kept away from heat sources. Because of their negligible vapor pressure, the ILs can be handled outside the fume hood. The wastes produced were discarded by the safety division of this Institute.
3. Results and Discussion
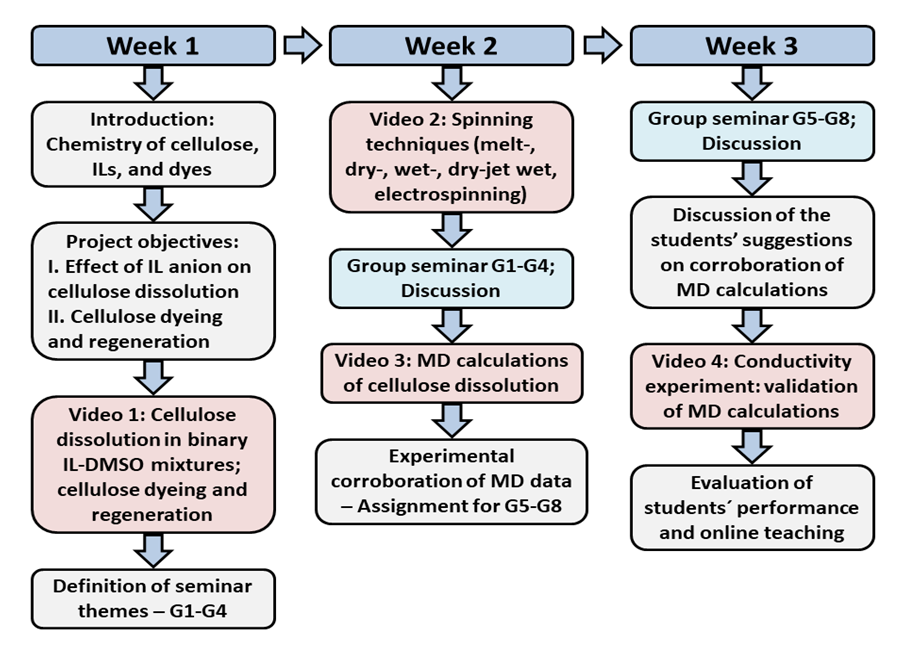
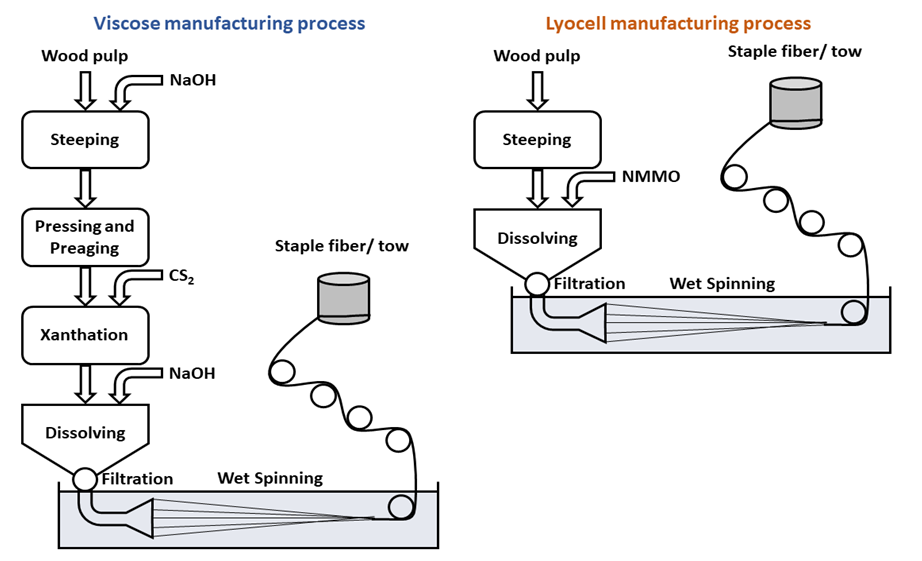
- We included the series of activities schematically depicted in Scheme 2 to enhance the students’ interest and participation in the online course. All course material was posted at the homepage that the University provides. The students communicated their assignments using Google Forms. During the first week, we introduced the objective/relevance of the project: use of green solvents (ionic liquids) to dissolve a renewable biopolymer, a step that is required for processing of Cel from sources other than cotton, and for recycling of, e.g., Cel and its blends with polyethylene terephthalate (PET) [5,8]. Whereas cotton can be spun directly, Cel from wood cannot; it must be dissolved and then regenerated in a suitable bath, e.g., as fibers and films [21]. This process occurs with formation- (chemical dissolution) or without formation of covalent bonds (physical dissolution). Industrial examples of both strategies are shown in Scheme 3 for the production of Viscose and Lyocell fibers, respectively.
3.1. Activities of the First Week (4 h)
- In conformity with the use of green solvents in carbohydrate chemistry, we used ILs to dissolve MCC, because of their efficiency, safety, and ease of recycling into the process [23,24]. We explained that the use of AlMeImCl and AlMeImAcO is intended to show how the efficiency of ILs as Cel solvents depends on the structure of the IL. We also explained that the use of a co-solvent, DMSO, is intended to decrease the biopolymer solution viscosity, hence enhance heat- and mass-transfer during MCC dissolution. Before regenerating the Cel film, we dyed the biopolymer, not only for aesthetic effect, but also to introduce textile dyeing, a subject that is rarely discussed in sufficient details, e.g., in organic chemistry courses, see Scheme 2. We then showed a video on: MW-mediated synthesis of AlMeImCl; IL purification; the transformation (AlMeImCl → AlMeImAcO) on ion-exchange resin; MCC dissolution using the equipment shown in Figure 1, and the dissolution criterion depicted in Figure 2; dyeing and regeneration of the biopolymer film, Figure 3. Because several of the subjects in this video are new to the students, we suggested a list of seminars to be given during the next class. The themes covered four seminars on the industrial processes for fabrication of the (commercial) Viscose, and Lyocell fibers, the newer (pilot plant-scale) Carbamate and Ioncell processes [25] and aspects of cellulosic fiber dyeing with reactive dyes (fixed to the fiber by covalent bonds) and by other dyes (fixed by adsorption), as well as tests for light- and washing fastness of dyes [26].
3.2. Activities of the Second Week (4 h)
- At the beginning of this class, we showed videos on the different spinning techniques [27-29], then four student groups (G1 to G4) gave the seminars assigned in the last class. After the seminars we showed the results of MD simulations of Cel dissolution. The objective of the first videos is to show a basic different between processing of Cel and synthetic polymers: the strategy of processing by extrusion from the melt, commonly applied to synthetic polymers, cannot be employed for Cel because the biopolymer thermally decomposes before melting [30].On the other hand, cellulose and its derivatives, e.g., esters can be dissolved, and the resulting solution is spun into filament. Depending on the volatility of the solvent, the fiber can be fabricated by dry spinning, where the solvent is evaporated by a stream of hot air, leaving the dry fiber (e.g., cellulose triacetate/acetone). In wet spinning, the fiber is regenerated by a chemical reaction (e.g., acid hydrolysis of Cel xanthate), or by dissolution of the Cel solvent (e.g., N-methylmorpholine-N-oxide) into the liquid in the bath (e.g., water, a non-solvent for Cel). The Viscose and Lyocell fibers are obtained by this approach, see Scheme 3 [31].
3.3. Activities of the Third Week (4 h)
- At the start of this online class, groups (G5-G8) presented their seminars on the assignment given last week. The students chose conductivity, FTIR, NMR, and dynamic light scattering as the experimental techniques. They were able to correctly show that the chosen techniques can be used to demonstrate the stronger association of Cel with AlMeImAcO. We showed the students our conductivity data, Figure 5 that depicts the dependence of solution conductivity on the molar ratio IL/OH group of cellobiose (a model for Cel). The conductivity of the cellobiose/IL-DMSO solution depends on the degree of dissociation of the IL, the mobility of the ions, as well as the strength of interactions cellobiose-IL ions. Because the contribution of the free IL cation to solution conductivity is the same for both ILs, then the difference between the two IL-DMSO in the absence of cellobiose must be due to more extensive dissociation of AlMeImAcO (17.280 mS cm-1) than AlMeImCl (8.900 mS cm-1). Note that the ionic mobility in water, at 40°C for the chloride ion is 2 times that of the acetate ion. That is, based on the ion mobility alone, the order of solution conductivity in the absence of cellobiose is expected to be: AlMeImCl > AlMeImAcO; this is not the case.
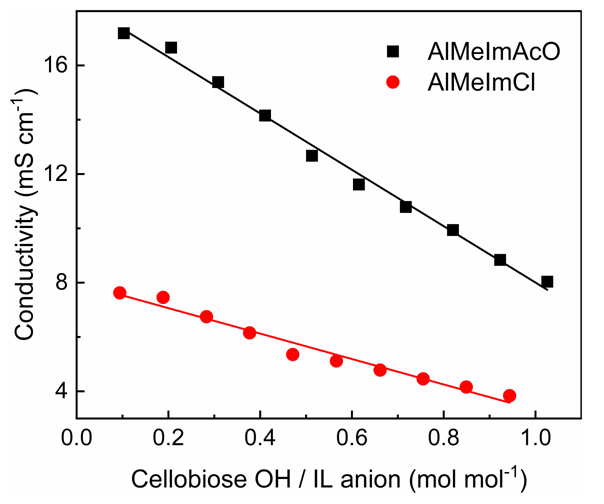 | Figure 5. Dependence of the conductivity of cellobiose solutions in AlMeImAcO and AlMeImCl-DMSO on the concentration of cellobiose, at 40°C |
3.4. Evaluation of Students’ Performance and Online Teaching
- The students were evaluated based on their presentation of the seminars, including their answers to the questions raised, and their participation during demonstrating the videos. The groups presented satisfactorily their seminars, although most of the subjects are new to them, e.g., dyeing mechanisms, spinning techniques and MD simulations. The students reported they learned new material and found the seminar themes interesting (80.9%). Regarding online teaching, 59.6% indicated that online classes did not replace face-to-face classes, whereas 66.0% thought that the activities given did not replace the experience acquired by doing the experiment. Regarding their local environment, 74.5% considered the conditions at home good/very good (internet connection and hardware). In spontaneous comments, the 85.1% of students appreciated our active learning approach and our efforts to produce the videos.
4. Conclusions
- Distance teaching of an experimental course is challenging because the students do not perform the experiment. To increase their interest, we introduced a project within the contexts of ESD and green chemistry: dissolution of Cel in green solvents (ILs-DMSO), followed by biopolymer regeneration as films. We used videos to show the experiments done, asked the students to give seminars related to the project, and to suggest an experiment to corroborate the results of MD simulations. An important gain of this project is showing the power of theoretical calculations to assess information that is not accessible experimentally (biopolymer chain separation). We recommend this project (distance-, or face-to-face teaching) for students of science courses (chemistry, engineering, pharmacy) because it is a nice example of ESD, and is safe (taken the proper precautions), low-cost, generates very little waste, and links theory to practice.A final remark: In 2020, we did not expect that teaching this same course would be shifted from face-to-face- to distance learning. In 2021 we were better prepared for online teaching, and we planned an experiment considering the new challenge. We kept the students interested/involved, thanks to the green chemistry approach, presentation of videos and seminars, and the assignment on MD simulations. There is an ongoing discussion about the advantages of using a hybrid teaching system in the future. Regarding experimental courses, after returning to face-to-face teaching, we will continue showing videos of the staff while carrying out the experiments, before going to the laboratory. This is a very useful approach to highlight important experimental precautions/protocols e.g., regarding the proper handling of chemicals and equipment.
ACKNOWLEDGEMENTS
- We are grateful to the students who participated in this project, Dr. Paulo Augusto Rodrigues Pires for help with molecular dynamics simulations, and Dr. Daniela C. Ferreira for providing the cotton powder. O. A. El Seoud thanks FAPESP (grant 2014/22136-4) and CNPq (grant 306108/2019-4) for financial support and for research productivity fellowship, respectively; N. Keppeler thanks CNPq for PhD fellowship (grant 141853/2019-0).
Abbreviations and Acronyms

 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML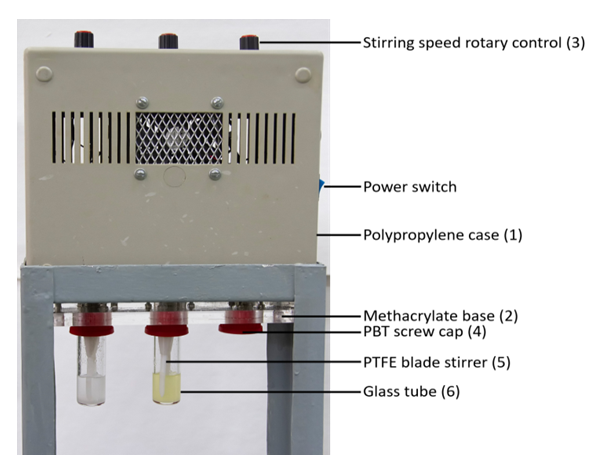
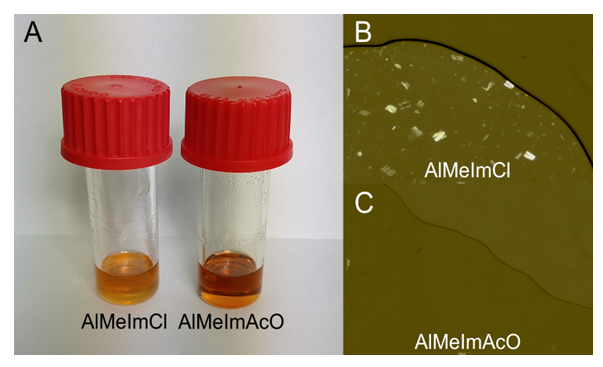
 = 0.6 and 70°C. The dissolution tubes (A) show partial- and complete MCC dissolution in AlMeImCl-DMSO (left) and AlMeImAcO-DMSO (right), respectively. The optical microscope images show the presence of undissolved MCC (B) in AlMeImCl-DMSO (upper image) and their absence (C) in AlMeImAcO- DMSO (lower image)
= 0.6 and 70°C. The dissolution tubes (A) show partial- and complete MCC dissolution in AlMeImCl-DMSO (left) and AlMeImAcO-DMSO (right), respectively. The optical microscope images show the presence of undissolved MCC (B) in AlMeImCl-DMSO (upper image) and their absence (C) in AlMeImAcO- DMSO (lower image)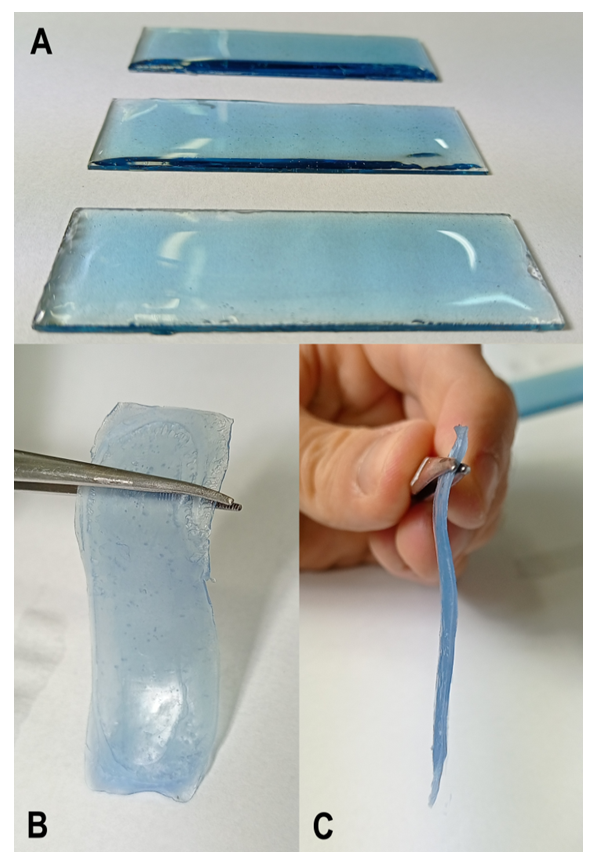
 = 0.6; at 70°C) for 30 minutes; added 5 drops of DBU and ca. 1 mg of Reactive Blue 2 reactive dye. The semi-transparent film regeneration was carried out as described above
= 0.6; at 70°C) for 30 minutes; added 5 drops of DBU and ca. 1 mg of Reactive Blue 2 reactive dye. The semi-transparent film regeneration was carried out as described above