Roch Chan-Yu-King, Jaclyn McCasland, Jose Medina, Jordan McBride, Bled N’Guessan, Melissa Donley, Veronica Owusu
University of Science and Arts of Oklahoma, Chickasha, OK, USA
Correspondence to: Roch Chan-Yu-King, University of Science and Arts of Oklahoma, Chickasha, OK, USA.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
We describe a simple, straight forward acid- base titration which makes use of readily available commercial reagents. The experiment can be incorporated into a curriculum suitable for high school or college level general, organic, analytical or upper-level inorganic chemistry laboratories. Unlike the traditional acid-base titration that calls for the addition of phenolphthalein as the indicator of the end point, this method uses 2,4-dinitrophenyl acetic as the analyte and indicator. The experiment can be used to illustrate or discuss the (a) concept of resonance in aromatic compounds, (b) use of Henderson-Hasselbach equation for the determination of Ka/pKa, (c) difference in acidity (Ka or pKa) between carboxylic acids, phenolic compounds or carbon acids, and (d) influence of anionic-charge delocalization via resonance on the generation of colored species in solutions.
Keywords:
High school chemistry, General chemistry, Organic chemistry, Analytical chemistry, Inorganic chemistry, Acid-base titration, Ka/pKa, Carbon acids, Resonance, Color of indicator
Cite this paper: Roch Chan-Yu-King, Jaclyn McCasland, Jose Medina, Jordan McBride, Bled N’Guessan, Melissa Donley, Veronica Owusu, Dual Role of 2,4-Dinitrophenyl Acetic Acid as Analyte and Indicator in Conventional Acid-Base Titration, Journal of Laboratory Chemical Education, Vol. 9 No. 1, 2021, pp. 15-20. doi: 10.5923/j.jlce.20210901.03.
1. Introduction
Acid-base titrations are ubiquitous chemical experiments found in high school or college undergraduate lab manuals. They are often carried out for the quantitative determination of a substance of unknown concentration. In a typical titration, the water-soluble acid analyte could be a weak organic (e.g., acetic acid) or strong inorganic acid (e.g., HCl) being neutralized with a strong titrant (e.g., NaOH) in the presence of an indicator such as phenolphthalein for the detection of the end point. The lab protocol often instructs the students to detect the end point by visual inspection and the titration is terminated when the solution turns from colorless to persistent pale pink. While the reaction kinetics of phenolphthalein with a base [1] and explanations to its color changes have been described [2], we found that most of the current commercial lab manuals do not cover its chemistry. This is presumably due to one of the following reasons: (a) the students have not taken any organic chemistry courses, (b) the first semester of college organic courses rarely covers the chemistry of aromatic compounds, (c) there is insufficient prelab instructional time to cover the topic, or (d) the instructor assumes that the students have already come across and understand the mechanism of reactions leading to the color changes. The molecule of phenolphthalein is relatively large with three six-carbon rings (two phenolic and one aromatic with a carboxyl group cyclized as a lactone) and thus, students often have difficulty memorizing the complete acid-base reaction sequence or mechanism (Figure 1). To avoid the use of phenolphthalein and still discuss the base-induced color changes of the indicator in titrations, we have decided to investigate the possibility of using smaller aromatic acids that can not only function as analytes, but also serve as their own indicators. 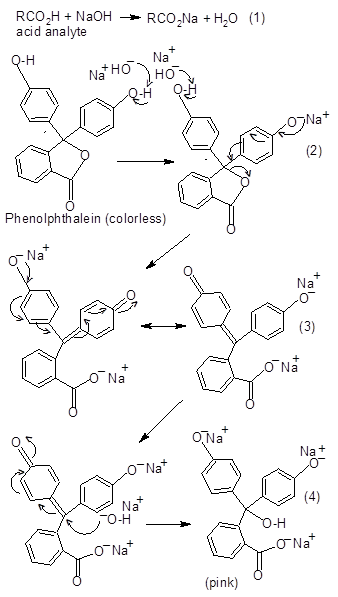 | Figure 1. Reactions of NaOH with a weak organic acid and phenolphthalein |
At our institution, we have consistently encouraged and enrolled undergraduate science majors in chemistry research. In the search of a phenolphthalein substitute, the student researchers were first given lectures on the determination of concentration of organometallics via titrations. For example, it is known that the determination of the molarity of strong bases such as Grignard reagents (RMgX) or alkyllithium (RLi) in anhydrous THF is often conducted with organic aromatic molecules functioning as the acid analytes as well as the indicators. Examples of these molecules may include salicylaldehyde phenyl hydrazine [3], N-benzyl benzamide [4] or diphenyl acetic acid [5]. In the case of the diphenyl acetic acid reaction with a titrant such as n-butyllithium or tert-butyllithium, the titrated lithium carboxylate solution remains colorless at the end point. However, further addition of RLi promotes the deprotonation of the benzhydryl-H (or α-H of the carboxyl group), forming a resonance stabilized dianion which yields a yellow solution (Figure 2). Since the RLi reacts with the carboxyl-H of a known amount of diphenyl acetic acid in equimolar ratio, the generation of the yellow color signals the end of the titration. Thus, the dual role of diphenyl acetic acid as analyte and indicator is clearly demonstrated in this experiment. The use of RMgX or RLi in high school or college undergraduate titrimetry labs may involve equipment issues for large size classes, budgetary constraints and safety consideration as RLi is corrosive and pyrolytic. Moreover, the experiments need to be carried out under anhydrous conditions in an inert atmosphere and at low temperature. With this information, the student researchers were instructed to investigate the feasibility of conducting the titration of diphenyl acid (or related aromatic acids) under safer conditions, i.e., at room temperature, in air and atmospheric pressure with NaOH(aq) as the base and water as the (co) solvent. These students were further instructed to select affordable analytes for titrations from chemical vendors (Sigma-Aldrich, Alfa Aesar, Acros Organics or Fisher Scientific). In addition, they were given the option to prepare an analyte using the available reagents from the chemical stockroom with the stipulation that the reaction is of high yield.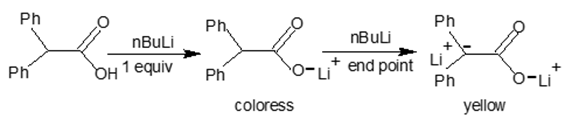 | Figure 2. Reaction of n-BuLi with diphenyl acetic acid as the indicator |
1.1. Search for the Potential Phenolphthalein Substitute as Analytes
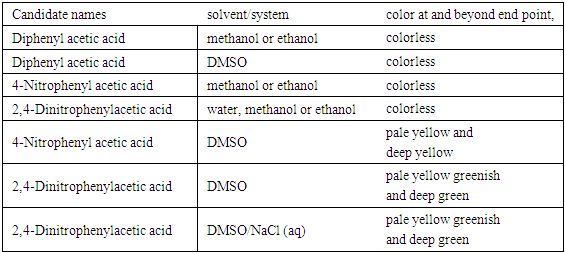
Preliminary results provide the candidate analytes, the solvents used and the observed color changes, if any, at and beyond the end point (Table 1). Most organic acids with six or more carbon atoms are sparingly soluble in water and the titrant (NaOH) is dissolved in water, the choice of the proper solvent(s) capable of dissolving both the organic acid and NaOH to form a homogeneous solution could be challenging. Solvents were selected from published procedures or commercial lab manuals on titrations of aromatic acids. They were either polar protic (water, alcohols), polar aprotic (DMSO) or a solvent system composed of NaCl/H2O/DMSO. It is worth mentioning that the students attempted to dinitrate diphenyl acetic acid with 70% conc. HNO3 (<10°C) hoping to use the product as analyte. The TLC (eluant: EtOAc/hexanes/EtOH, 10/20/1, vol/vol/vol) of the deep orange oily crude product (which did not crystallize out on cooling) indicated a mixture of UV sensitive compounds. When a sample of this mixture (dissolved in DMSO) was treated with NaOH (aq), a deep orange reddish color resulted. Subsequent flash liquid chromatography (using the same eluant as above) of the reaction mixture failed to yield a major pure product. The titration of 2,4-dinitrophenyl acetic acid (2,4-DNPAcOH) with NaOH (aq) (Table 1) gave a discrete color change from colorless to pale yellow greenish (Figure 3) and then to deep green (Figure 4)) making it the “model analyte”. The color change was the same whether using aqueous solution of DMSO [6] or DMSO and NaCl (aq) [7], so the latter solvent system was selected to homogenize the aromatic analyte. The reaction of 2,4-DNPAcOH with NaOH (aq) is displayed in Figure 5. Table 1. Potential phenolphthalein substitutes
 |
| |
|
 | Figure 3. Color of titrated 2.4-DNPAcOH solution at the end point |
 | Figure 4. Color of titrated 2,4-DNPAcOH solution beyond the end point |
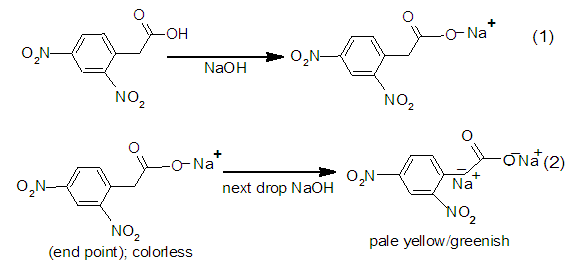 | Figure 5. Reaction of 2,4-DNPAcOH with NaOH |
2. Experimental
2.1. Chemicals and Hazards
All chemicals are purchased from Alfa Aesar or Sigma Aldrich Co. and used as received with the exception of 2,4-DNPAcOH which was recrystallized in ethanol. A new bottle of DMSO was used as cosolvent.Students must wear goggles and gloves during lab time and avoid coming into direct contact with 2,4-DNPAcOH (skin, eye and lung irritant), NaOH (a corrosive base) and DMSO (absorbable by skin).
2.2. Pre-lab Preparation
A lab assistant prepares an NaCl (aq) stock solution (concentration:1g NaCl/100 ml DI water) and a stock solution of NaOH (aq) (concentration between 0.10 to 0.15M). Prior to prelab lecture, the lab assistant titrates a solution of 2,4-DNPAcOH in NaCl(aq)/DMSO and takes pictures of the titrated solutions at and beyond end point. These pictures are projected on a screen during the lab session to give the students a color reference. The titration can be completed during a three-hour lab period.
2.3. Procedure
Using an analytical balance, weigh out accurately between 0.200 to 0.230g of recrystallized 2,4-DNPAcOH, record the weight and transfer the acid into a 50 ml clean beaker containing a small magnetic stir bar. Then add 10 ml DMSO and stir until the analyte is completely dissolved. This is followed by the addition of 6 ml NaCl (aq) with stirring. Fill a clean 50 ml burette with the NaOH (aq) solution and titrate the stirred solution by adding ca. 0.50 ml NaOH (aq) at a time (slightly exothermic) until an approximate volume of 6.0 ml is added. Continue the addition of the titrant in about 0.2 ml increments. The end point is reached when the entire solution turns from colorless to a persistent pale yellow greenish color (Figure 3) which should last at least 25-30 seconds. The observation of a deep green color (Figure 4) indicates that the titration has gone past the end point. Record the total volume of NaOH (aq) needed to reach the end point. Repeat this experiment at least two more times. Each analyte sample should have a mass between 0.200 to 0.230g but should be different from that used in the previous titrations.
2.4 Objectives: Identification of an Unknown Acid Analyte via its Equivalent Mass or Molar Mass and Determination of the Unknown pKa
As part of their weekly lab work, students enrolled in the second trimester of organic chemistry (Spring 2018) and general chemistry (Spring 2019) at our institution were given 2,4-DNPAcOH as an “unknown” analyte for titration. They are to carry out a conventional acid-base titration with NaOH(aq) as titrant and compute the equivalent mass (EW) of the unknown using the equations (1) and (2) below:(1) EW = (mass of the unknown acid used)/(MNaOH X VNaOH), where MNaOH is the molarity of the base and VNaOH the volume in liters of the titrant recorded at the end point. (2) EW = MM/number of titratable hydrogen(s).Using the computed EW, the students will deduce the corresponding molar mass (MM) of the unknown and match it with that of a molecule given in the following list of possible (monoprotic or diprotic) analytes:a) Monoprotic acids and the corresponding MM (g/mol): Formic acid (46.03); Acetic acid (60.05); Benzoic acid (122.12); Phenyl acetic acid (136.15); 4-Nitrobenzoic acid (167.12); 4-Nitophenyl acetic acid (181.15); Diphenyl acetic acid (DiPhAcOH) (212.25); 2,4-DNPAcOH (226.14). b) Diprotic acids and the corresponding MM (g/mol): Oxalic acid (90.03); Malonic acid (104.07); Succinic acid (118.09); Adipic acid (146.14); Phthalic acid (166.14).
3. Results and Discussion
3.1. Conventional Acid-Base Titration
A total of twenty- four students, working in pairs, carried out the experiment. Figure 6 shows a typical volume of titrant vs pH curve. Each pair of students conducted at least three titrations and reported the corresponding molar masses (MM, g/mol), their mean average () and the unknown analyte name. Using the provided by the students, we have computed their MM percent error (%) with respect to that of the pure 2,4-DNPAcOH (226.14g/mol). These results along with the corresponding standard deviations (σ) are given in Table 2. Eight pairs of students who obtained above 3 % MM error selected DiPhAcOH as the unknown and four pairs (whose MMs are below 3%) selected 2,4-DNPAcOH. Much like in the traditional weak acid-strong base titrations conducted in the presence of phenolphthalein whose color turns from colorless to pale pink (end point) and then to deeper pink (beyond the end point), the students’ judgement of the corresponding pale yellow greenish color in this titration is subjective and is most likely the main source of experimental error. The data in Table 1 indicate that the presence of DMSO as a solvent component is required for the appearance of color at the end point and the acid analyte must have at least a nitrated aromatic ring. The latter moiety highly stabilizes the α-carbanion of the analyte and helps induce a bathochromic shift (from colorless to colored) of the titrated solution. While the source of color observed remains to be investigated, the possibility of a charge transfer between the resonance stabilized α-carbanion and DMSO (which contains a weak pπ-dπ bond [8]) cannot be ruled out since precedents of donor-acceptor processes involving DMSO and Tetracyanoethylene have been reported [9]. Several points are also worth mentioning to the students: the sequence of deprotonations in Figure 1 shows the relative acidity of the substances involved, i.e., the analyte carboxyl-H (step 1) is more acidic (higher Ka) than the phenolic-OHs (lower Ka) of the indicator. In addition, Figures 2 and 5 indicate that the carboxyl-Hs are more acidic than the α-CH of the carboxyl groups. Students enrolled in or have taken the second semester of the traditional organic chemistry course sequence know that carbonyl containing compounds, having α-CH(s), such as aldehydes, ketones, esters, carboxylic acids and their derivatives are acidic towards NaOH since their α-carbanions are stabilized by delocalization. Therefore, the acidity of DiPhAcOH and 2,4-DNPAcOH should not be surprising to them. Incidentally, the definition of “carbon acids” could be introduced to the students and used to describe DiPhAcOH, 2,4-DNPAcOH because their acidic-Hs are directly attached to the α-carbon atom of the carboxyl group.Table 2. Results from conventional titrations
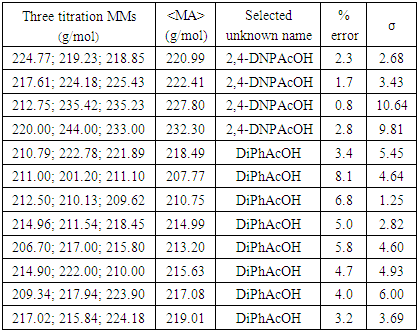 |
| |
|
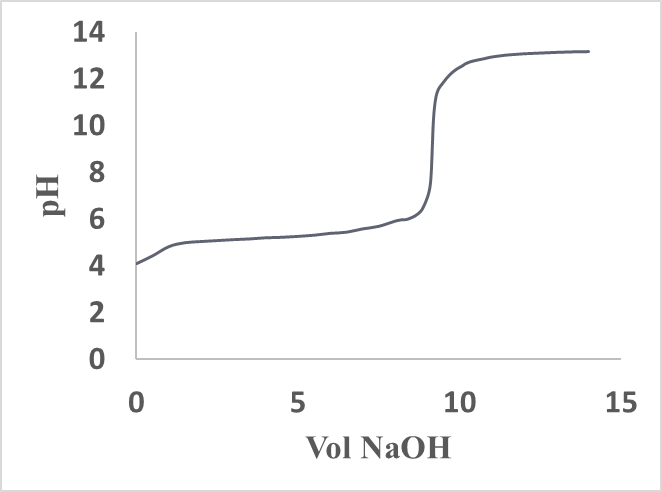 | Figure 6. A typical 2,4-DNPAcOH-NaOH (aq) titration curve |
3.2. Potentiometric Titration
Using pre-calibrated pH meters, the student researchers conducted a total of 10 titrations. Sample of selected data is found in Table 3 along with Figure 7 displaying the first derivative: (ΔpH/ΔV) vs. the average volume (Vavg) of NaOH used. In addition, Table 4 gives values (around the end point zone, starting from ca.8.0 to 11.0 ml) needed to plot the corresponding second derivative (Fig. 8). This plot is obtained by placing (1) on the y-axis, the values of Δ(ΔpH/ΔV)/ ΔVaverage (which relates the rate of change of the first derivative to the change in the average volume), and (2) on the x-axis, the values of the average of two successive volumes used for the first derivative plot (V’avg) The point where the curve passes through the x-axis provided the equivalence volume (9.15 ml). To the best of our knowledge, no experimental pKa of 2,4-DNPAcOH has been reported even though a theoretical value of 3.2 has been predicted in water [10]. It is worth pointing out that the determination of the effect of solvent system and ionic strength on pKa values is quite complex and its discussion is beyond the scope of this work [11]. Nevertheless, the students have estimated the pKa of the analyte using the equivalence volume derived from the second derivative plot and the Hendersen-Hasselbach equation. Their results are as follows: 5.22, 5.40, 5.35, 5.42, 5.20, 5.20, 5.30, 5.37, 5.10 and 5.35 (mean pKa: 5.29).Table 3. Vol. NaOH (aq) vs. pH and first derivative data
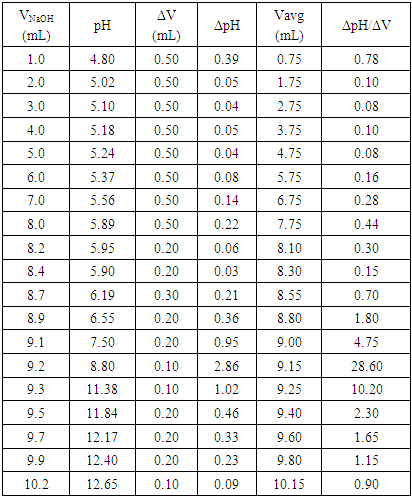 |
| |
|
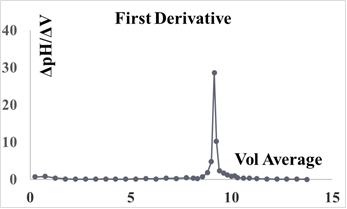 | Figure 7. First derivative: ΔpH/ΔV vs Vavg of NaOH (aq) |
Table 4. Data used to plot the second derivative
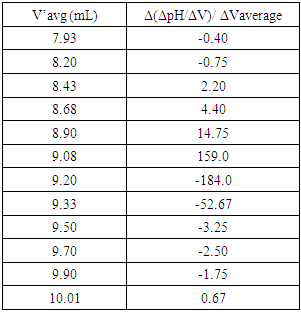 |
| |
|
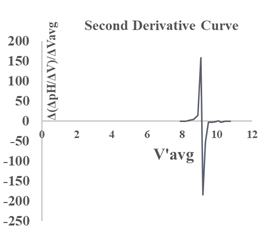 | Figure 8. Second derivative:Δ(ΔpH/ΔV)ΔVavg vs V’avg of NaOH (aq) |
4. Conclusions
One of the traditional high school or college undergraduate lab experiments often involves the titration of a weak organic acid with a strong base. Such titrations make use of two organic molecules: the acid analyte and phenolphthalein as the indicator. In this paper, we have demonstrated the feasibility of using only one organic molecule, the 2,4-dinitrophenyl acetic acid which functions not only as analyte, but also as its own indicator. Two groups of students enrolled in General Chemistry II and Organic Chemistry II labs were given 2,4-DNPAcOH as an unknown for titration. They successfully carried out the experiment and determined the unknown molar mass, via the equivalent weight formula, with satisfactory % errors. Depending on the student backgrounds, the simple experiment can be used to introduce the definition of “carbon acids” and illustrate various basic chemistry concepts or principles such as the effects of resonance on the stabilization of carbanions and the subsequent generation of color change (e.g., a bathochromic shift) and the relative acidity (pKa or Ka) of a carboxyl-H, phenolic-OH, methylene or methine-H located at the α-position of a carbonyl group.
ACKNOWLEDGEMENTS
The authors thank the Foundation of the University of Science and Arts of Oklahoma-Gladys Anderson Emerson research fund for financing the project and the students enrolled in Organic chemistry II lab (Spring 2018) and General chemistry II lab (Spring 2019) for carrying out the titrations. In addition, the authors are grateful to Dr. Jeanette M. Loutsch for her help in proofreading the manuscript.
References
| [1] | Wittke, G., 1983, Reaction of phenolphthalein at various pH values., J. Chem. Ed., 60 (3), 239-240. |
| [2] | Nicholson, L., 1989, Kinetics of the fading of phenolphthalein in alkaline solution., J. Chem. Ed., 66 (9), 725-726. |
| [3] | Love, B.E., and Jones, E. G., 1999, The use of salicylaldehyde phenylhydrazone as an indicator for the titration of organometallic reagents., J. Org. Chem., 64 (10), 3755–3756. |
| [4] | Burchat, A. F., Chong, J. M., and Nielsen, N., 1997, Titration of alkyllithiums with a simple reagent to a blue endpoint., J. Organomet. Chem., 542, 281-283. |
| [5] | Kofron, W.G., and Baclawski, L. M., 1976, A convenient method for the estimation of alkyllithium concentrations., J. Org. Chem., 41, 1879-1880. |
| [6] | Budesky, O., 1989, Effectivity of solvents-a new approach in non-aqueous acid-base titrimetry., Talenta., 36(12), 1209-1216. |
| [7] | Helmkamp, G. K., and Johnson, H. W., 1983, Selected experiments in organic chemistry, 3 ed., W. H. Freeman and Company: New York/San Francisco, pp.169-170. |
| [8] | Reuter, H., 2017, Structural parameters of dimethyl sulfoxide, DMSO, at 100 K, based on a redetermination by use of high quality single-crystal x-ray data., Acta Cryst. E., 73 (10), 1405–1408. |
| [9] | Steward, F. E., Eisner, M., and Carper, W. R., 1966, Study of DMSO-TCNE charge transfer complex and kinetics., J. Chem. Phys., 44, 2866-2872. |
| [10] | Abibi-Yangjeh, A., and Danandeh-Jenagharad, M., 2007, Prediction of acidity constant for substituted acetic acids in water using artificial neural networks, Ind. J. Chem., 46(B), 478-487. |
| [11] | Reijenga, J. C., Van Hoof, A. J. F., Van Loon, A. H. M., and Tunissen, A. J. P., 2013, Development of methods for the determination of pKa values, Anal. Chem. Insights, 8: 53–71. |


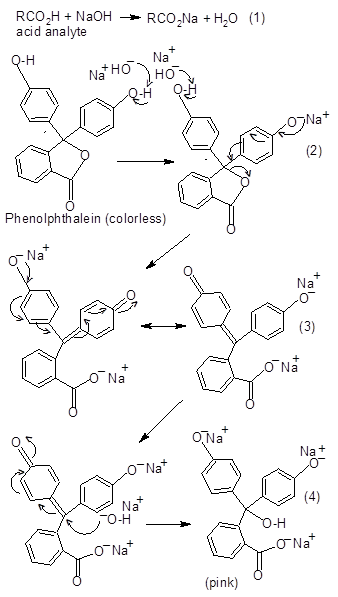







 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML


