Simon Cassegrain , Wen Zhou , Nabyl Merbouh
Department of Chemistry, Simon Fraser University, 8888 University Drive, Burnaby, BC, V5A
Correspondence to: Nabyl Merbouh , Department of Chemistry, Simon Fraser University, 8888 University Drive, Burnaby, BC, V5A.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
While case studies have found extensive uses in many disciplines, they are still lacking in chemistry. This paper aims to introduce two NMR spectroscopy case studies, an opportunity to showcase that chemistry could be taught using this format. Starting from simple isomeric molecules and their full spectroscopic analyses, students will be asked to unequivocally identify their structures as well as label all the spectra provided, all while being exposed to the intricacies of solving a problem set compiled in a case study format.
Keywords:
Case Study, NMR spectroscopy, Organic Chemistry, Arylnaphthalene Lactones, Undergraduate students, Isomers
Cite this paper: Simon Cassegrain , Wen Zhou , Nabyl Merbouh , The Arylnaphthalene Lactones Tale: Two NMR Spectroscopy Case Studies on the Surprises of a Dehydro-Diels−Alder Reaction, Journal of Laboratory Chemical Education, Vol. 9 No. 1, 2021, pp. 10-14. doi: 10.5923/j.jlce.20210901.02.
1. Introduction
In our previous work we aimed to expose undergraduate students to small NMR spectroscopy and mass spectrometry challenges using simple, ubiquitous and readily available structures. Students learned about the use of HMBC to help differentiate between 3-Phenylbutyric acid (1) and α-methylhydrocinnamic acid (2), [1] used NMR spectroscopy and mass spectrometry to differentiate between 3-chlorochalcone (3) and 3’-chlorochalcone (4), [2] or between cis-5-Norbornene-endo-2,3-dicarboxylic anhydride (5) and cis-5-Norbornene-exo-2,3-dicarboxylic anhydride (6) using 1D NOE and NOESY experiments. [3] But if teaching examples such as the ones described above are easily found, their format however remains the same: Here are the spectra - Figure out the structure. A very dry and often unappealing format. A format that many other disciplines such as medicine, business or biology to name few have moved away from to focus on a more enticing case-based approach. A well-documented approach that has shown many benefits. [4] A case tells a story, is learner centered and more importantly ropes the reader into a story and a path to self-learning that might not be possible under other examination formats. [5]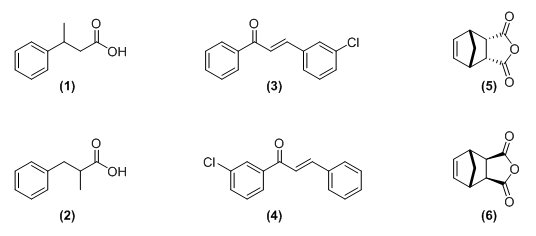 In this manuscript we will expose the students to two NMR spectroscopy centered case studies involving a student trials and tribulations during his first month of graduate studies. The story of this student is authentic, and is designed to engage all students in order to reach a solution either individually or as a group. The students will be given several analytical tools to help reach a solution, and through a careful read of the cases and discussions with their instructors or group, will be asked to solve as much of the cases as possible. In the process, the students should be encouraged to acquire and develop analytical, collaborative, as well as communication skills, in order to disseminate their results appropriately. This is also a chance for instructors to connect students with real life data, drawn from their own experience whenever possible, a great exposure to the challenges they will soon face.
In this manuscript we will expose the students to two NMR spectroscopy centered case studies involving a student trials and tribulations during his first month of graduate studies. The story of this student is authentic, and is designed to engage all students in order to reach a solution either individually or as a group. The students will be given several analytical tools to help reach a solution, and through a careful read of the cases and discussions with their instructors or group, will be asked to solve as much of the cases as possible. In the process, the students should be encouraged to acquire and develop analytical, collaborative, as well as communication skills, in order to disseminate their results appropriately. This is also a chance for instructors to connect students with real life data, drawn from their own experience whenever possible, a great exposure to the challenges they will soon face.
2. Case Study # 1. The Hare and the Tortoise: Slow and Steady Gets You the Correct Target!
It was early September 2020 when Taylor a first-year master’s student started an exciting project synthesizing arylnaphthalenes derivatives. His first targets, two arylnaphthalene lactones were selected for their antibacterial and antiviral properties. More importantly, Taylor was tasked with making each arylnaphthalene lactones as a Type 1 and a Type 2 scaffold (Scheme 1). [6]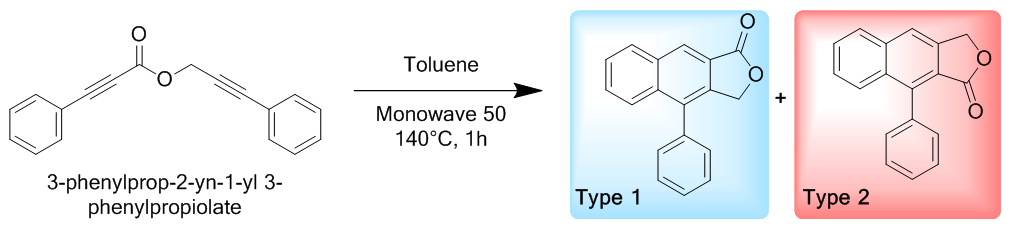 | Scheme 1. The possible structures |
Taylor. Taylor is a 21 years old student who just graduated with a bachelor in organic chemistry. As a Co-op student his interest was piqued by medicinal chemistry and structure–activity relationship (SAR) studies of synthetic drugs. So needless to say, that when he was assigned the arylnaphthalenes project he did not think twice before accepting. Taylor is a very task oriented and energetic student who does not shy from hard work and long working hours. However, at times Taylor can be impatient and rushes synthetic steps in hope of getting to his final targets a little faster or with fewer steps. Arylnaphthalene Lactones. Arylnaphthalene lactones are naturally occurring compounds in many medicinal plants. This subclass of lignans is reported to exhibit antibacterial, antiviral, antitumor activities only to name few. The most common synthetic routes to access them use either an intramolecular Diels–Alder (DA) reaction of 3-arylprop-2-en-1-yl-3-arylpropiolate followed by an aromatization step or a dehydro-Diels−Alder (DDA) reaction of 3-arylprop-2-yn-1-yl-3-arylpropiolate. [7] The Dehydro-Diels−Alder Reaction. [4+2] Cycloadditions also called Diels-Alder (DA) reactions have been widely studied since their discovery in 1928. These reactions occur by the thermal activation of a diene with a dienophile leading to a 6-membered ring formation in a one-step concerted mechanism. Many variations of DA reactions have been investigated and recently a new type of DA reactions have been described: the Dehydrogenative Diels-Alder (DDA). In a DDA, components of the reaction could be an alkene or an alkyne. This reaction is particularly useful in the production of naphthalene derivatives than can be readily obtained by the reaction of a styrene scaffold moiety with an alkyne moiety. [8]The Synthesis. After a closer look at the literature on arylnaphthalene lactones, Taylor decided to use the 3-arylprop-2-yn-1-yl-3-arylpropiolate (3-phenylprop-2-yn-1-yl 3-phenylpropiolate) synthetic route to build his Type 1 and Type 2 scaffolds in one go. He also elected to use his Monowave 50 oven activation in order to cut down on lengthy reaction times and hopefully shorten the number of steps on the way to his final targets. He set his eye on a representative reaction in which he reacted 3-phenylprop-2-yn-1-yl 3-phenylpropiolate in an intramolecular DDA. Using Toluene as a solvent, Taylor irradiated his ester for one hour at 140°C. After the irradiation step, an excited Taylor was able to isolate two UV active products with Rfs of 0.2 and 0.4 in 10% ethyl acetate/hexane respectively. [9]The Issue. After a long day in the laboratory, Taylor’s excitement was short lived as he quickly realized that both compounds had the same mass spectrum, and similar IR and 1H-NMR spectra. To make matters worse, in his excitement, Taylor forgot to label his round bottom flasks properly resulting in a mix-up. With, both solids in hand and before leaving for the day, Taylor submitted an NMR full package (1H-NMR, 13C-NMR, APT, COSY, HSQC and HMBC) on each compound in hope to be able to unequivocally identify his products the next day. [2]The Spectra. The next morning, with a full set of spectra on hand, Taylor found a calm spot in the library and started investigating the results of his analyses. Will he be able to figure out what he made, and more importantly will he be able to explain it to his peers during his scheduled laboratory meeting in two days. Will he be needing additional analyses to further proof his structure? And more importantly, was the DDA synthetic route a viable route to pursue for his project.A Light-Bulb Moment. While immersed in his multitude of spectra, Taylor suddenly remembered his spectroscopy class and his introduction to NOE spectroscopy and the possibility to observe through space couplings. Can these extra NMR experiments (NOESY or 1D-NOE) help him with his spectral assignment? Unfortunately, with time pressing and the busy spectrometers, Taylor could only carve out 30 minutes of magnet time to run a couple of 1D-NOE experiments. It was decision time for Taylor, which protons should he irradiate in order to optimize his time and get the answers he is looking for? [3]Questions. Given all the spectra recorded by Taylor could you help him with his structures’ elucidation? Could you assist him unequivocally assign all the peaks in his 1H-NMR and 13C-NMR spectra?
3. Case Study # 2. Can Slow, Steady and Green Get You the Correct Target?
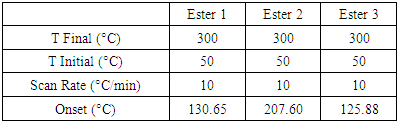
The day after his successful group meeting, and with a fresh motivation, Taylor decided to tackle his arylnaphthalene lactones synthesis using a different and more selective strategy. Taylor was unequivocally able to identify and fully characterize the Type 1 and Type 2 arylnaphthalene lactones he synthesized starting from 3-arylprop-2-yn-1-yl-3-arylpropiolate. However, the lack of selectivity of this synthetic route made him lean toward a different route where he will be able to selectively synthesize a single Type only. [6] Arylnaphthalene Lactones and the Dehydro-Diels−Alder Reaction (DDA). As it turns out each type of arylnaphthalene lactones (Type 1 and type 2) could be selectively synthesized using a Dehydro-Diels−Alder reaction on unsaturated esters: 3-phenylprop-2-yn-1-yl cinnamate derivatives for Type 1 and cinnamyl 3-phenylpropiolate derivatives for Type 2. [7,8]Preparing for the Syntheses. In his typical efficient fashion Taylor quickly synthesized both cinnamyl 3-phenylpropiolate (Ester 1) and 3-phenylprop-2-yn-1-yl cinnamate (Ester 2). However, this time Taylor with the help of his analytical chemistry instructor took the time to run a differential scanning calorimetry (DSC) for each ester before choosing the irradiation temperature. This was triggered by Taylor remembering an article he read where the DSC investigation of a thermally induced cyclization was able to provide insights and understanding of the reaction. [10]The DSC Results. Taylor managed to run the DSCs on all his esters (Table 1), even the one from his first experiment, 3-arylprop-2-yn-1-yl-3-arylpropiolate (Ester 3), and compile the results in the table below. This gave him a good idea for the irradiation temperature ranges he needed to investigate for his DDA reactions, which allowed him to save a lot of time, energy and starting material.Table 1. Summary of the DSC results for ester 1, 2 and 3
 |
| |
|
Arylnaphthalene Lactones Synthesis. Using the DSC data collected Taylor chose a common solvent to be used for all his DDA reactions. His choice was toluene as it will allow him to heat all his reactions at temperatures ranging from 130°C all the way to 240°C, while staying within the allowed pressure of his Monowave 50 oven instrument. Using, toluene as his solvent, Taylor was able to easily synthesize the desired Type 1 and Type 2 lactones from 3-phenylprop-2-yn-1-yl cinnamate at 240°C and cinnamyl 3-phenylpropiolate at 140°C respectively, a true feat especially since both transformations were quantitative within few hours of reaction time. The Solvent Switch. With both arylnaphthalene lactones and a set of reliable and reproducible syntheses on hand, Taylor decided to turn his attention to the Type 2 lactones synthesis. His first order of business was a solvent switch. The reason, Taylor has learned during his co-op that not all solvents where used in industry and that most of the solvent were ranked as follow: recommended, problematic, hazardous or even highly hazardous. In all the surveys Taylor consulted, toluene was often considered problematic or hazardous while ethanol another potential solvent for his DDA reactions was always considered preferred or recommended. He immediately, decided the switch from toluene to ethanol, especially since his DDA only required a temperature of 140°C for the formation of Type 2 arylnaphthalene lactones, temperature that ethanol and his Monowave 50 oven was able to handle without any issues. [11]An Important Solvent Effect. Upon heating cinnamyl 3-phenylpropiolate at 140°C in ethanol for 1 hour, Taylor observed an interesting phenomenon. After, cooling the reaction to room temperature, beautiful crystals become forming almost quantitatively. Phenomenon not observed when toluene was used as a solvent (Scheme 2). After filtering and drying these needle shaped crystals, a quick 1H-NMR left him puzzled: this was not the expected Type 2 arylnaphthalene lactone. So, what was it? Can a simple switch in solvent lead to a different reaction outcome? Is it possible? [12]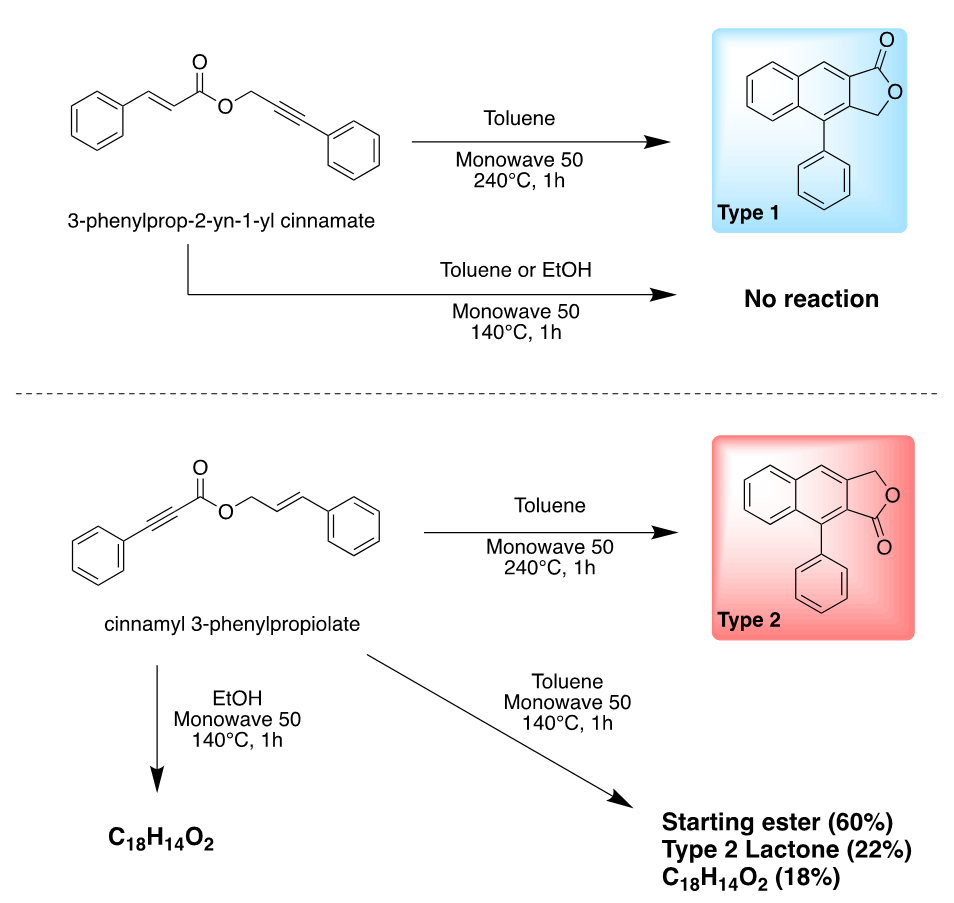 | Scheme 2. Solvent effect summary |
The Spectra. Taylor needed to sort this solvent effect as soon as possible. What did he make and could he figure out the structure of this compound. This time Taylor requested an NMR full package (1H-NMR, 13C-NMR, APT, COSY, HSQC, HMBC and NOESY) an recorded an IR spectrum as well as a mass spectrometry spectrum in order to solve this new structure. [2]Questions. Given all the spectra recorded by Taylor could you help him solve the structure of his new compound? Could you help him unequivocally assign all the peaks in the 1H-NMR and 13C-NMR spectra?
4. Conclusions
Case studies are designed to trigger discussions and more importantly a quest for self and guided learning. Both cases were designed with the following outcomes in mind: 1) teach the students to use critical thinking when reading a case, 2) sieve through all the unnecessary facts and only focus on the relevant and needed information in the text, 3) avoid getting overwhelmed with the large amount of analytical data provided, but rather learn the strength and limitations of each analytical analysis: what is this spectrum telling me, 4) often you need more than one analysis to prove your structure, 5) other students might adopt a different strategy to solve the case and still get to the same conclusion, 6) accept the challenge and seek help, this is a good training for later. This is also a great opportunity for instructors to use their own experience to generate such cases with new and authentic material. Furthermore, with the global shift to remote learning and teaching during the COVID-19 pandemic, the use for new material and new ways to engage and motivate students is essential and what better way than to start with a story.
ACKNOWLEDGEMENTS
The authors would like to thank the Simon Fraser University Dean of Science Office and Chemistry Department for their generous financial support.
Appendix
SI -Full - studentsCase Studies Instructors SISI Full Instuctors
References
| [1] | Woo, E. H. S.; Field, M. J.; Sutherland, M. L.; Gerak. C. A. N.; Merbouh, N. A simple case study for the introduction of the HMBC and the EI-MS techniques to second and third year undergraduate students. The Chemical Education Journal (CEJ), 2017, 18 (33). http://www.edu.utsunomiya-u.ac.jp/chem/v18n1/101Merbouh/Merbouh.html (Last accessed February 2021). |
| [2] | Gerak, C. A. N.; Sutherland, M. L.; Field, M. J.; Woo, E. H. S.; Merbouh, N. The use of a small chalcone spectroscopy database for the introduction of advanced spectroscopy techniques at the undergraduate level. Chem. Educ. 2016, 21, 119-128. |
| [3] | Sannikov, O.; Ye, E.; Pinto, B. M.; Saunders, P.; Merbouh, N. Introducing Complex NMR Mixtures at the Undergraduate Level: Isomerization, Separation and Analysis of the Diels-Alder Adducts from the Reaction of Methylcyclopentadiene and Maleic Anhydride (Part II)” J. Lab. Chem. Educ, 2020 8(3), pp. 39-80. DOI: 10.5923/j.jlce.20200803.01. |
| [4] | Graham, A. (2011) Making the Case: Writing and Using Case Studies for Teaching and Knowledge Management in Public Administration. Queen's Policy Studies Series (Vol. 148). ISBN-10: 1553393023. |
| [5] | Herreid, C. F. (2007). Start with a story: The case study method of teaching college science. NSTA Press. ISBN-10: 1933531061. |
| [6] | Zhao, C.; Rakesh, K. P.; Mumtaz, S.; Moku, B.; Asiri, A. M.; Marwani, H. M.; Manukumar, H. M.; Qin, H-L. Arylnaphthalene lactone analogues: synthesis and development as excellent biological candidates for future drug discovery. RSC Adv. 2018, 8, 9487-9502. DOI: 10.1039/c7ra13754k. |
| [7] | (a) Kocsis, L. S.; Brummond, K. M., Intramolecular Dehydro-Diels-Ader Reaction Affords Selective Entry to Arylnaphthalene or Aryldihydronaphthalene Lignans. Org. Lett. 2014, 16, 4158-4161. DOI: 10.1021/ol501853y. (b) Saavedra, D. I.; Rencher, B. D.; Kwon, D. H.; Smith, S. J.; Ess, D. H.; Andrus, M. B. Synthesis and Computational Studies Demonstrate the Utility of an Intramolecular Styryl Diels Alder Reaction and Di-t-butylhydroxytoluene Assisted [1,3]-Shift to Construct Anticancer dl-Deoxypodophyllotoxin. J. Org. Chem. 2018, 83, 2018-2026. DOI: 10.1021/acs.joc.7b02957. (c) Li, J.-H.; Tang, J.-S.; Xie, Y.-X.; Wang, Z.-Q.; Deng, C.-L., Phosphazene Base-Catalyzed Intramolecular Cascade Reactions of Aryl-Substituted Enynes. Synthesis 2010, 18, 3204-3210. DOI: 10.1055/s-0030-1258172. |
| [8] | Wessig, P.; Müller, G. The Dehydro-Diels-Alder Reaction. Chem. Rev. 2008, 108, 2051–2063. DOI: 10.1021/cr0783986. |
| [9] | Rajesh, U. C.; Losovyj, Y.; Chen, C-H.; Zaleski, J. M. Designing Synergistic Nanocatalysts for Multiple Substrate Activation: Interlattice Ag−Fe3O4 Hybrid Materials for CO2‑Inserted Lactones. ACS Catal. 2020, 10, 3349−3359. DOI: 10.1021/acscatal.9b04260. |
| [10] | Höhne, G. W. H.; Hemminger, W.; Flammersheim, H.-J. (2003) Differential scanning calorimetry: an introduction for practitioners. Berlin; New York: Springer. ISBN 978-3-662-06710-9. |
| [11] | Prat, D.; Hayler, J.; Wells, A. A survey of solvent selection guides. Green Chem., 2014, 16, 4546-4551. DOI: 10.1039/c4gc01149j. |
| [12] | Cativiela, C.; García, J. I.; Mayoral, J. A.; Salvatella, L. Modelling of solvent effects on the Diels–Alder reaction. Chem. Soc. Rev., 1996, 25, 209-218. DOI: 10.1039/CS9962500209. |


 In this manuscript we will expose the students to two NMR spectroscopy centered case studies involving a student trials and tribulations during his first month of graduate studies. The story of this student is authentic, and is designed to engage all students in order to reach a solution either individually or as a group. The students will be given several analytical tools to help reach a solution, and through a careful read of the cases and discussions with their instructors or group, will be asked to solve as much of the cases as possible. In the process, the students should be encouraged to acquire and develop analytical, collaborative, as well as communication skills, in order to disseminate their results appropriately. This is also a chance for instructors to connect students with real life data, drawn from their own experience whenever possible, a great exposure to the challenges they will soon face.
In this manuscript we will expose the students to two NMR spectroscopy centered case studies involving a student trials and tribulations during his first month of graduate studies. The story of this student is authentic, and is designed to engage all students in order to reach a solution either individually or as a group. The students will be given several analytical tools to help reach a solution, and through a careful read of the cases and discussions with their instructors or group, will be asked to solve as much of the cases as possible. In the process, the students should be encouraged to acquire and develop analytical, collaborative, as well as communication skills, in order to disseminate their results appropriately. This is also a chance for instructors to connect students with real life data, drawn from their own experience whenever possible, a great exposure to the challenges they will soon face.

 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML