-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2020; 8(2): 28-32
doi:10.5923/j.jlce.20200802.02
Received: July 21, 2020; Accepted: August 2, 2020; Published: August 29, 2020

A Novel Test for Qualitative Detection of Carbohydrates
Sangeeta Pandita
Department of Chemistry, Zakir Husain Delhi College, J.L. Nehru Marg, New Delhi, India
Correspondence to: Sangeeta Pandita, Department of Chemistry, Zakir Husain Delhi College, J.L. Nehru Marg, New Delhi, India.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The contemporary chemistry teaching laboratories endeavour to integrate the current themes of safety, sustainability and simplicity in all aspects of experimental design. The qualitative analysis of carbohydrates is central to study of organic chemistry and biochemistry laboratory courses at undergraduate level. This paper presents a novel qualitative test for detection of carbohydrates using a single reagent, alkaline potassium hexacyanoferrate (III). This reagent shows a characteristic behaviour towards carbohydrates. The observation is developed and presented as a simple, safe and easy to perform test for detection of carbohydrates. The test is suitable for micro scale as well as mini scale quantities. It involves no special technique to perform, is quick to accomplish and gives unambiguous results. These attributes make it suitable to be adopted in teaching laboratories.
Keywords: Alkaline potassium hexacyanoferrate (III) reagent, Carbohydrates, Qualitative test
Cite this paper: Sangeeta Pandita, A Novel Test for Qualitative Detection of Carbohydrates, Journal of Laboratory Chemical Education, Vol. 8 No. 2, 2020, pp. 28-32. doi: 10.5923/j.jlce.20200802.02.
Article Outline
1. Introduction
- Carbohydrates are a fascinating class of biomolecules having unique structural features that distinguish them from other classes of organic compounds and also form the basis of their characteristic reactions [1,2]. It is due to this special aspect that carbohydrate analysis is fundamentally somewhat different from systematic qualitative analysis of other classes of organic compounds. Molisch test [3,4] is used to establish the presence of carbohydrates. This test requires the use of concentrated sulphuric acid which is to be poured into an aqueous solution (safety hazard) using a special technique. Also, the test gives difficult to interpret results if the concentration of carbohydrate and that of Molisch reagent (alcoholic solution of α- naphthol) are not in proper proportions. These aspects usually make it difficult for freshmen students to achieve results without ambiguity.In order to devise a simple protocol that poses no safety hazard, is easy to perform and gives unambiguous results, our attention was drawn to alkaline potassium hexacyanoferrate (III) oxidation of carbohydrates which has been studied in literature from several aspects including kinetic, qualitative and quantitative aspects [5-7]. Alkaline potassium hexacyanoferrate (III) has been used in our laboratory to afford clear distinction among different classes of carbohydrates [7]. During the course of the reaction, it was observed that carbohydrates cause disappearance of the greenish-yellow colour of the reagent to colourless state. This behaviour was found to be characteristic of carbohydrates. Other classes of commonly available organic compounds either caused no disappearance of colour or gave darker colours due to oxidation under test conditions. Disappearance of colour was observed to happen only in case of carbohydrates. In this paper, the observed unique behaviour of carbohydrates is developed and presented as a novel test for qualitative detection of carbohydrates as a class of organic compounds.
2. Experimental Design
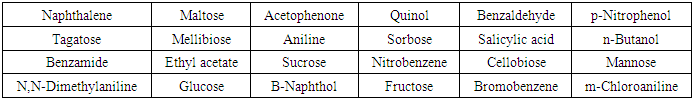
- The experimental design of this study takes into account the choice of compounds, the methodology for accomplishing the test, ease of performing the test and clarity in interpreting the test results. Methodology has been detailed in the next section under the title Experimental Details. The choice of compounds was based on the type of compounds undergraduate students usually receive during qualitative organic analysis laboratory. These are listed in Table 1. Carbohydrates chosen are monosaccharides (aldoses and ketoses) and disaccharides (reducing and non reducing). Other compounds in Table 1 are representatives of carboxylic, carbonyl, ester, amino, substituted amino, nitro, alcoholic, phenolic and amide functions.
3. Experimental Details
- 3a. Preparation of alkaline potassium hexacyanoferrate (III) reagentThe reagent used in this test is 20%/1% alkaline potassium hexacyanoferrate (III). It is prepared by dissolving 20 g NaOH pellets in 100 mL distilled water carefully. It generates a large amount of heat and should be cooled to surrounding temperature for the next step. Solid potassium hexacyanoferrate (III) (1g) is then added and dissolved to give a solution that has a greenish-yellow colour. This is the alkaline potassium hexacyanoferrate (III) reagent used in the study. It has a shelf life of two weeks at ambient laboratory temperatures. It can be stored in clear glass bottles, preferably away from acid bottles as the reagent is not compatible with acids. 3b. Test procedureA mixture of unknown compound (20 mg solid or two drops of liquid compound) and 1 mL alkaline potassium hexacyanoferrate (III) reagent is taken in a test tube, shaken gently and warmed to about 60°C in a water bath. Decolourization is observed within 1-5 minutes in case of carbohydrates.For micro scale quantities of the unknown compound, the test can be performed as per the following procedure:A groove tile is thoroughly cleaned and rinsed with distilled water. 0.1-0.2 mg of the test compound is placed in the groove. 3-4 Drops of warm alkaline potassium hexacyanoferrate (III) reagent (preheated to about 60°C in water bath) is now added. Gentle stirring with a fine tipped glass stirring rod is continued until disappearance of colour is observed.
4. Observations and Results
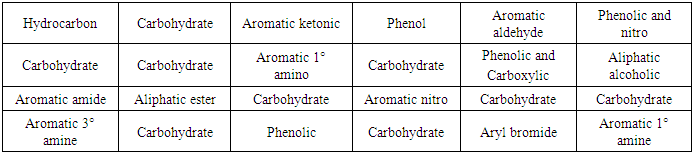
- The observations of the reaction of alkaline potassium hexacyanoferrate (III) reagent with carbohydrates and organic compounds containing various functional groups are presented in Table 1. Pictures are also presented for visual correlation. Figures 1 and 2 are for micro scale test in groove tiles while Figure 3 shows test results performed in test tubes. The results are easy to interpret as carbohydrates show as colourless solutions.
|
|
 | Figure 1. Alkaline potassium hexacyanoferrate (III) test of functional groups soon after mixing |
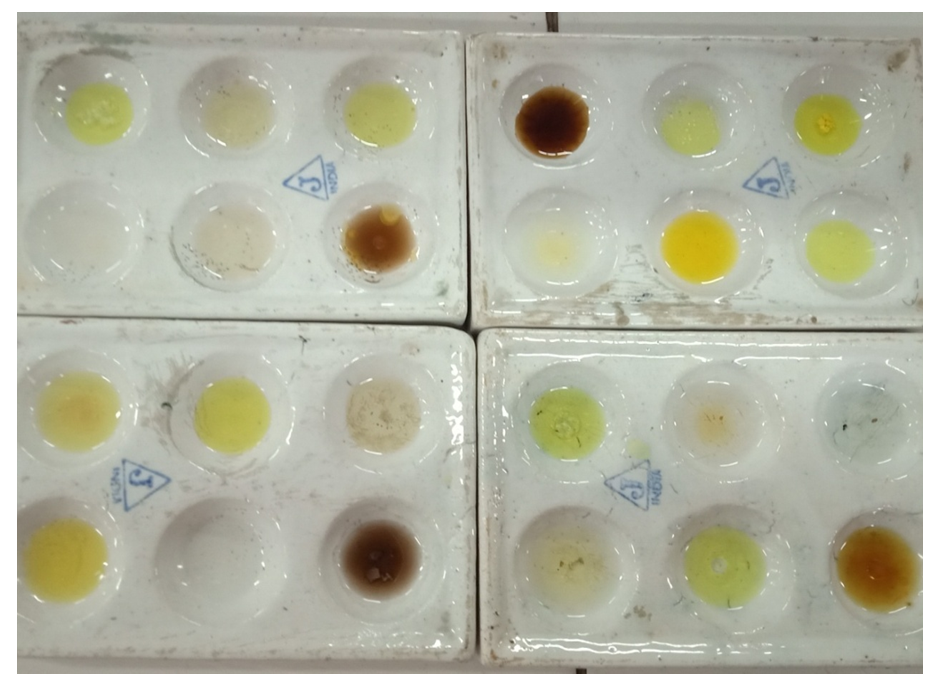 | Figure 2. Alkaline potassium hexacyanoferrate (III) test of functional groups: final colours |
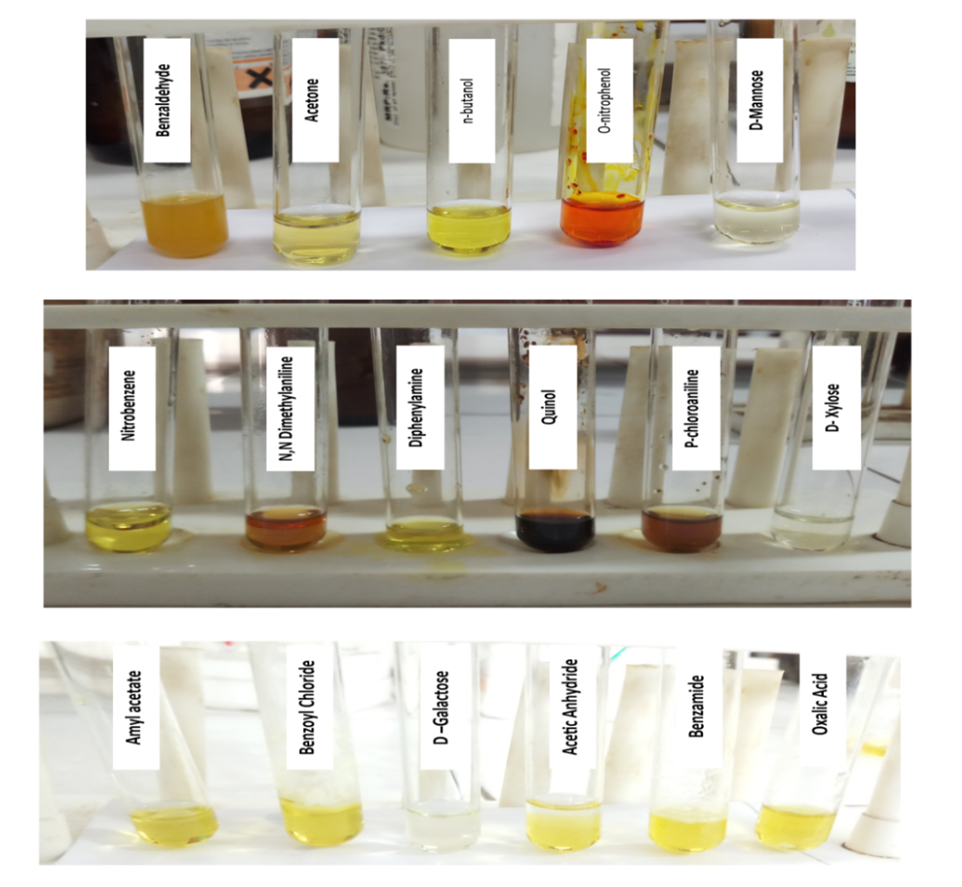 | Figure 3. Test at milligram scale. Carbohydrates can be identified easily as colourless solutions in each group |
5. Discussion
- Hexacyanoferrate (III) is known to be an efficient single electron oxidant for a wide variety of organic substrates such as, reducing sugars, phenols, naphthols, aromatic amines, phenolic aldehydes etc. The CN− ligands being resistant to substitution reactions under these conditions, the preferred pathway of oxidation is the outer-sphere electron transfer pathway [8,9]. Therefore, oxidations are generally clean with fewer side reactions. A general reaction may be formulated as:
 Oxidation of sugars by alkaline potassium ferricyanide has been studied in detail [5-7] and it has been found that the reaction is first order both with respect to alkali and carbohydrate and zero order with respect to ferricyanide [6]. Thus, if either the concentration of carbohydrate or alkali or both is increased, the decolourization happens faster. Several concentrations of alkali were tried and 20% was found to give results that students are able to observe properly and interpret with ease.From the results presented in Figures 1-3, it is apparent that carbohydrates can be easily identified by their ability to decolourize the alkaline potassium hexacyanoferrate (III) reagent. Aromatic amines and phenols are known to get oxidized by alkaline potassium hexacyanoferrate (III) to give a mix of oxidative coupling products, quinine imines and quinones which are highly coloured due to extended conjugation [10]. Thus, under oxidizing conditions of the test reagent, amines and phenols show darker colours. Other compounds like carboxylic acids, esters, amides, nitro compounds etc. already have sufficiently oxidized state of carbon and therefore are not oxidized further and cause no change in the colour of the reagent. Thus, these compounds retain the greenish- yellow colour, making the identification of carbohydrates clear and unambiguous.The alkaline potassium hexacyanoferrate (III) test presented here uses no special technique. It involves mixing the test compound and the reagent and observing the disappearance of the colour. Students find it easy to perform and interpret.
Oxidation of sugars by alkaline potassium ferricyanide has been studied in detail [5-7] and it has been found that the reaction is first order both with respect to alkali and carbohydrate and zero order with respect to ferricyanide [6]. Thus, if either the concentration of carbohydrate or alkali or both is increased, the decolourization happens faster. Several concentrations of alkali were tried and 20% was found to give results that students are able to observe properly and interpret with ease.From the results presented in Figures 1-3, it is apparent that carbohydrates can be easily identified by their ability to decolourize the alkaline potassium hexacyanoferrate (III) reagent. Aromatic amines and phenols are known to get oxidized by alkaline potassium hexacyanoferrate (III) to give a mix of oxidative coupling products, quinine imines and quinones which are highly coloured due to extended conjugation [10]. Thus, under oxidizing conditions of the test reagent, amines and phenols show darker colours. Other compounds like carboxylic acids, esters, amides, nitro compounds etc. already have sufficiently oxidized state of carbon and therefore are not oxidized further and cause no change in the colour of the reagent. Thus, these compounds retain the greenish- yellow colour, making the identification of carbohydrates clear and unambiguous.The alkaline potassium hexacyanoferrate (III) test presented here uses no special technique. It involves mixing the test compound and the reagent and observing the disappearance of the colour. Students find it easy to perform and interpret. 6. Safety Instructions
- The dissolution of NaOH pellets in water is an exothermic reaction. This step requires careful slow dissolution with frequent cooling to ambient laboratory temperature. Sodium hydroxide solution is corrosive and should never be handled with bare hands. Potassium hexacyanoferrate (III) in alkaline medium has very low toxicity and is a mild irritant to eyes and skin. The experiment should be performed with usual personal protective equipment, i.e., lab coat, gloves and safety goggles.
7. Conclusions
- A novel test based on the observation that carbohydrates display a unique behaviour towards alkaline potassium hexacyanoferrate (III) has been developed for qualitative detection of carbohydrates. The test is easy and safe to perform, and quick to accomplish. It uses only two chemicals, namely, sodium hydroxide and hexacyanoferrate (III). Both these chemicals are inexpensive and easily available in basic chemistry teaching laboratories.No special technique or skill is required to perform the test. The results of the test are easy to interpret; greenish-yellow to colourless means carbohydrate is present. Carbohydrates can, thus, be detected easily, unambiguously. The complete protocol including reagent preparation and test procedures developed can be conveniently adopted by teaching laboratories.
ACKNOWLEDGEMENTS
- Gratefully acknowledge the authorities at Zakir Husain Delhi College (ZHDC), New Delhi, India, for providing the necessary laboratory facilities for Students’ Summer Research Project-2019. Participation of Dr.Jyoti Tyagi in the project is acknowledged with thanks. Achyut Ranjan Gogoi and Iftikhar Hussain, students of B.Sc. Chemistry Honours (batch 2016-19) of ZHDC, carried out some testing experiments required in this project. Their contribution is acknowledged.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML