-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2020; 8(1): 18-22
doi:10.5923/j.jlce.20200801.04

Reduction of a Ketone by Sodium Tetrahydridoborate – A Comprehensive Experiment of Common Laboratory Techniques for Organic Chemistry Reaction
Tsz-Wing Wong
Department of Chemistry, Pui Ching Middle School, Hong Kong, China
Correspondence to: Tsz-Wing Wong, Department of Chemistry, Pui Ching Middle School, Hong Kong, China.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Hands-on laboratory experiences are critical for students in the learning process in all areas of study, especially for science education. Laboratory experiments are part of the science curriculum. It is no doubt that hands-on activities play an irreplaceable role in scientific education. Hands-on experiments are the key of learning science and also make the science lessons more attractive. The practical activities are essential for learning chemistry and enhancing science literacy. Through hands-on chemistry experiments, students can investigate chemical properties and reactions through utilizing laboratory apparatus and instruments. Common laboratory techniques in organic chemistry include synthesis, isolation, purification and characterization of the desired product. Reflux, simple distillation, liquid-liquid extraction and recrystallization are the basic training of experimental skills. In organic chemistry experiments, students are required to obtain pure product from reactants through a number of steps. However, we found that in many fundamental organic chemistry experiments, only one or two common laboratory techniques are involved. In this article, we will demonstrate an experiment of student self-learning pre-laboratory preparation integrated with a comprehensive approach of using common laboratory techniques in organic reaction. Besides, we aim to train our students to be an active learner and also increase their self-confidence and independence on conducting experiments. We designed a 2-hour laboratory session in which serval common laboratory techniques are arranged systematically so that students can manipulate the techniques and also conduct analysis for identification of product.
Keywords: Reduction of ketones, LiAlH4, NaBH4, Basic laboratory techniques
Cite this paper: Tsz-Wing Wong, Reduction of a Ketone by Sodium Tetrahydridoborate – A Comprehensive Experiment of Common Laboratory Techniques for Organic Chemistry Reaction, Journal of Laboratory Chemical Education, Vol. 8 No. 1, 2020, pp. 18-22. doi: 10.5923/j.jlce.20200801.04.
Article Outline
1. Introduction
- Practical laboratory have been known as important central role in science curriculum [1] and provide a platform for students to apply and consolidate the knowledge delivered from didactic lectures and experience necessary practical skills for further research and employment. [2] [3] [4]Unfortunately, due to the reform of curriculum and the limitation of teaching time, the science teaching is usually a lecture-mode and focused on the delivery of knowledges and facts. The traditional laboratory experience becomes ‘cookbook’ experiments in which students follow the fixed procedure and then prepare the scientific report or complete a worksheet. In this situation, students do not have enough experience on manipulating the common laboratory skills. Moreover, if students do not prepare themselves in advance and not understanding the underlying principle and design of the practical laboratory, the effectiveness of laboratory activities has usually been queried [5] [6] [7]. In recent years, many have argued that if there are no valuable practical experiences in science curriculum, science is not meaningful to students. [1]
1.1. Reduction of Carbonyl Compounds
- Reduction of aldehydes and ketones is an important process in nature and it is also an important topic in any introductory Organic Chemistry course. In biological system, the similar reductions of aldehydes and ketones by NADH or NADPH are catalyzed by alcohol dehydrogenases.Typical reducing agents used in organic experiments are lithium tetrahydridoaluminate (LiAlH4) or sodium tetrahydridoborate (NaBH4). LiAlH4 was discovered by Finholt, Bond and Schlesinger in 1947. [8] It is used commonly in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides to give corresponding alkanols. This colourless solid is dangerously reactive toward water, releasing hydrogen gas (H2) and it is not recommended to store in school laboratory. The discovery of the reducing powers of NaBH4 occurred accidentally by Prof. H.C. Brown. [9] NaBH4 is relative mild, alcohols can be chosen as solvents. As LiAlH4 is sensitive to air and moisture and therefore dry ether is chosen as solvent. NaBH4 is a relatively mild reducing agent and it is less reactive than LiAlH4. NaBH4 is commonly used in the reduction of carbonyl compounds [10] but it cannot reduce an ester group, a carboxyl group and amide. It has a good efficacy on reducing aldehydes and ketones. The commonly used solvents include alcohols, tetrahydrofuran (THF), dimethylformamide (DMF) and water.In organic synthesis, the reactants react with appropriate reagents in different steps. Each step of a synthesis involves a chemical reaction, and reagents and conditions for each of these reactions must be designed to give an adequate yield of pure product.
1.2. Reflux
- Many organic chemical reactions take very a long period for completion. As reaction rate increases with temperature, it is very common to heat the reaction mixture in order to speed up these reactions. However organic compounds and solvents are often volatile with high vapour pressures and low boiling points. When heated to a certain temperature, they will evaporate and become flammable. Heating under reflux involves heating the reaction mixture for a specific amount of time. Meanwhile the solvent and volatile organic compounds evaporate and become vapour. With using a condenser, the hot vapour produced above the surface of the reaction mixture continually undergo condensation and return to the flask as a condensate. In this way, the reaction mixture can be heated for a long period and ensure the reaction can be completed.
1.3. Distillation
- Distillation is a purification method for liquids with different boiling points and it can separate components of a mixture if they have significantly different boiling points (differ from each other about 25°C). In a distillation, the liquid is boiled in a round-bottom flask or pear-shaped flask. The hot vapour moves to another part of the apparatus, the condenser, where they come into contact with a cool surface. The condensed liquid (the distillate) drips into a container separated from the original liquid. However, simple distillation cannot be used to separate the components in azeotropes.
1.4. Recrystallization
- Recrystallization is used as a purification technique for solids. By dissolving impure solid in a minimal amount of appropriate hot solvent, the hot solution is then allowed to cool slowly. The developing crystals ideally form with high purity while impurities remain in the mother liquor. The crystallized solid, i,e, crystals, is then filtered away from the impurities. In this way, one solvent is used and is called single-solvent recrystallization. In multi-solvent recrystallization, two or more solvents are used. In this process, by adding a less-soluble solvent to the hot mother liquor, crystals will be formed slowly as the solubility decrease gradually.
1.5. Liquid-Liquid Extraction
- Liquid-liquid extraction is also known as solvent extraction or partitioning. It is a separation process in which the solute is transferred from one solvent to another solvent. The two solvents are immiscible or partially miscible with each other. The basic principle is based on their relative solubilities in two different immiscible liquids. The ratio of the solute partitioning in two solvents depends on the phase equilibrium of the solute in two phases.
2. Reaction
- The mechanism of the reduction is well studied. [11] [12] NaBH4 is a source of hydride (H-) and the BH4- ion first attacks the carbon atom of the carbonyl group. Upon addition of water or acid, the oxygen is protonated to give the corresponding alcohol as in (1).
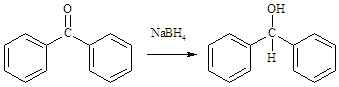 | (1) |
3. Experimental
3.1. Materials
- Benzophenone, sodium tetrahydridoborate solids (should be stored in desiccator), propan-2-ol, 10% sodium hydroxide solution, dichloromethane, 2,4-dinitrophenylhydrazine solution, concentrated hydrochloric acid, 0.1 M zinc chloride solution, hexane, anhydrous sodium sulphate solid. All other chemicals were of reagent grade and were used without further purification.
3.2. Apparatus
- Quick-fit apparatus, 250 mL separating funnel, 50 cm3 conical flasks.
3.3. Precautions
- NaBH4 solids react with water to give hydrogen gas which is flammable, propon-2-ol is flammable, dichloromethane is harmful.
3.4. Experiment
3.4.1. Reaction of Benzophenone with NaBH4
- In this experiment students worked individually. 1.0 g (5.5 mg) of benzophenone solids and 0.3 g (7.9 mg) of NaBH4 solids were added into a 50 cm3 dry pear-shaped flask respectively. 10 cm3 propan-2-ol was then poured in it. A few pieces of anti-bumping granules was added to the reaction mixture. The reaction mixture was heated under reflux for five minutes. The mixture was then cooled to room temperature.
3.4.2. Extraction of Product
- 10 cm3 of 10% NaOH(aq) solution was added into the resulting solution. The solution was transferred into a separating funnel. The pear-shaped flask was rinsed with 10 cm3 of water. The rinsing water was poured into the separating funnel. The product was then extracted with two portions of 8 cm3 of dichloromethane successively. The organic layer was collected in a dry conical flask.Warning: Release the gas pressure from separating funnel occasionally when shaking with dichloromethane.
3.4.3. Obtain Raw Product by Simple Distillation
- The organic layer was dried with anhydrous sodium sulphate solid. The solution was transferred into a 50 cm3 round-bottomed flask. The organic solution was then heated and dichloromethane was removed as distillate. A white wax-like solid was left in the round-bottomed flask as the crude product.
3.4.4. Recrystallization
- The crude product was dissolved in a minimum amount of hot hexane. The hexane solution was then heated until the crude product was completely dissolved. The hot hexane solution was divided into two portions. One portion was cooled slowly at room conditions while the other portion was placed into an ice-water bath for cooling rapidly. Two portions of crystals with different appearance were collected.
3.4.5. Analysis of Products
- Three chemical tests were conducted to compare the functional group of reactant and product.(a) Acidified KMnO4 solution(b) Lucas reagent (c) 2,4-dinitrophenylhydrazine solutionCheck the melting points and record the infra-red spectrum of the reactant and product.
4. Discussion
- In past few decades, the laboratory activities were planned to promote the student undertaking investigation and scientific inquiry. [13] Tobin (1990) [14] stated that: “Laboratory activities appeal as a way to learn with understanding and, at the same time, engage in a process of constructing knowledge by doing science” (p. 405). He also suggested that meaningful learning is possible in the laboratory session if students are given appropriate opportunities to manipulate equipment and materials as a result their knowledge of phenomena and related scientific concepts can be constructed.In recent years, the Senior Form science curriculum in Hong Kong has been reformed. Both teaching time of content and the time allocated for practical are reduced significantly. Under the stress of teaching time limitation, many science teachers plan their teaching focusing mainly on the content of textbooks. Although the practical contributes part of the overall grade of science subjects in the public examination, the requirements on skills and number of practical sessions are dramatically decreased. Science education is not only learning the basic concepts but should also stimulate the curiosity, to develop the skill of observations and to interoperate the data. [15] However, follow the teaching schedule is always challenging for teachers under the limitation of teaching time and therefore teachers have no choice to trim the practical or even only arrange a minimum numbers of experiment. It is no doubt that the weighing of practical as well as the common laboratory techniques becomes less important. Over past few decades, hands-on performance assessment has become a standard assessment format for measuring scientific inquiry competence [16] We believe that students can acquire deeper understanding of the concept through a well-organized practical laboratory especially in organic chemistry. Also, the well-trained of manipulating of common laboratory skills can make it faster and easier for students to master advanced and complex experimental skills in science disciplines of universities.Effective learning is not only listening, students should learn actively and zealously. Under the thread of time limitation of teaching and learning, we believe that students learn common laboratory skills more effective through self-advanced learning. Some students may find that observing the skills demonstrated by teachers or tutors in laboratory session may not helpful. It may due to the limited facilities of laboratory or the large class size. Previous study on self-directed learning in laboratory has shown that the self-motivated learning has a positive impact and meaningful contribution to chemistry success, self-directed learning readiness in laboratory and chemistry laboratory anxiety of students. [17] Videos of demonstrating the operation of common laboratory techniques such as simple distillation, reflux, handling of separation funnel are recorded and uploaded to school server. Students are asked to complete a pre-lab worksheet after watching the video and submit prior attending the laboratory session. The questions of the pre-lab worksheet included the general precaution of using separating funnel, the purpose of adding anti-bumping granules and drawing the experimental set-up of simple distillation etc. Besides, create the flexibility for students to learn on their own schedule and pace is also concerned. In this experiment, excess NaBH4 was used to ensure all the benzophenone was reduced. The percentage yield of diphenylmethanol is about 68%. Although the product diphenylmethanol can be collected as solid from resulting mixture after the reaction is completed, the purity is not high enough. In our approach, we used dichloromethane to extract the product from the aqueous reaction mixture. The boiling point of product diphenylmethanol (b.p. = 298.0°C) is higher than that of dichloromethane (b.p. = 39.6°C) and we used simple distillation to remove the solvent dichloromethane. As diphenylmethanol is polar and is not soluble in nonpolar solvent, we recrystallized the product with hot hexane. The solid product obtained by cooling slowly at room conditions for about 1 week is white large needle-shaped crystals while a white powder is obtained in rapid cooling process. We found that there were no crystals formed in few samples of students after serval days. It may due to the hexane solution is not concentrated enough. By adding extra portion of hexane into the mixture and heated until boiling, large crystals were obtained after cooling. The product gives a very sharp melting point (m.p. = 68.0°C). In the chemical tests, the hydroxyl group in diphenylmethanol undergoes oxidation with acidified KMnO4 and the solution turns from purple to colourless. In Lucas reagent test, the hydroxyl group is substituted by the chlorine and milky solution is observed as positive result. Only benzophenone reacts with 2,4-dinitrophenylhydrazine to give orange-red solid because the C=O bond reacts to produce C=N imine compound. All these simple chemical tests can be conducted with using test tubes and warmed in hot water bath.Compare the two infra-red spectra, there is a strong board absorption peak at 3320 cm-1 which indicates the presence of the hydroxyl group in product while there is no absorption peak at 1660 cm-1 which is responsible for the C=O bond. In this experiment, students can also experience the skill for recrystallization with using volatile organic solvent and notice the effect of the rate of recrystallization on shape of crystal formed.There were around 90 students of grade 11 in each academic year. Due to the limitation of equipment and laboratory space, maximum of 18 students can be accommodated in each session. We also arranged two teachers and one laboratory technician to provide a better supervision and sufficient guidance. Students were asked to conduct the experiment individually and completed the worksheet after conducting the analysis for benzophenone and diphenylmethanol. In the worksheet, students were required to do comparison on the results of the chemical tests and infra-red spectra of both reactant and product. From the pre-lab worksheets submitted by students before attending the experimental session, we found that the concepts of using common laboratory techniques were not clear and sometimes had misconceptions. For example, many students did not understand the function of adding anti-bumping granules in heating under reflux of volatile organic solvents and the reason why the valve of separating funnel should be opened occasionally while shaking.Through this experiment, the confidence of students in handling common laboratory techniques with many apparatus was significantly enhanced. It was especially obvious in the upcoming laboratory sessions. [18] Moreover the concept of most of the students on chemical properties of typical functional groups was consolidated.
5. Conclusions
- Benzophenone was treated with NaBH4 and was reduced readily to form diphenylmethanol. In this experiment, we presented an experiment consisting of a series of common laboratory techniques. Also students can gain a comprehensive experience in handling the basic skills for organic chemistry reactions.
ACKNOWLEDGEMENTS
- The work described in this paper was financially supported by Pui Ching Middle School. We thank for the guidance from the former Vice Principal Mr Lei Keng Lon and the technical support by the Chemistry panel members of Pui Ching Middle School.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML