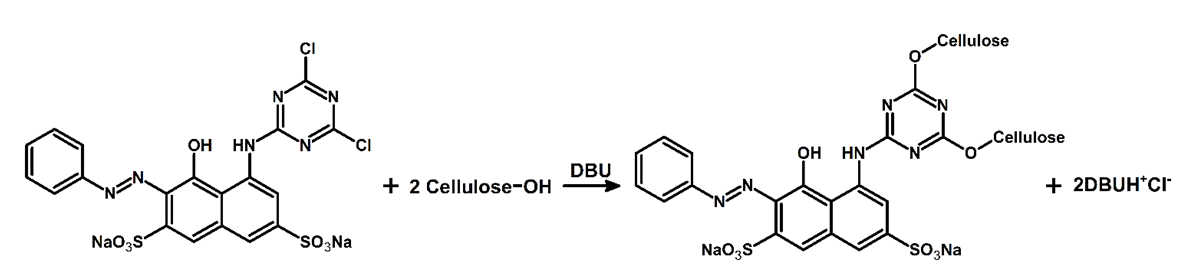
-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2020; 8(1): 11-17
doi:10.5923/j.jlce.20200801.03

Education for Sustainable Development: An Undergraduate Chemistry Project on Cellulose Dissolution, Regeneration, and Chemical Recycling of Polycotton
Omar A. El Seoud, Nicolas Keppeler
Institute of Chemistry, the University of São Paulo, São Paulo, Brazil
Correspondence to: Omar A. El Seoud, Institute of Chemistry, the University of São Paulo, São Paulo, Brazil.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

We introduced an undergraduate chemistry project within the framework of education for sustainable development (ESD). The objective of the first part was to demonstrate the efficiency of an ionic liquid (IL)- 1-(n-butyl)-3-methylimidazolium acetate (BuMeImAcO)- as solvent for the dissolution, and subsequent regeneration of cellulose as fiber. Under mechanical stirring, the students dissolved microcrystalline cellulose in a mixture of BuMeImAcO-dimethyl sulfoxide (DMSO) at 80°C. Subsequently, they dyed the dissolved cellulose with a reactive dye, and regenerated (pink) colored fibers by injecting the resulting biopolymer solution into water (a non-solvent for cellulose). The objective of the second experiment was to show the potential application of BuMeImAcO-DMSO for the chemical recycling of the cellulosic component of polycotton (cellulose: polyethylene terephthalate; PET). Cellulose dissolves under the above-mentioned experimental conditions, leaving a mat of PET. The students dyed the dissolved cellulose, and then regenerated the biopolymer as fiber. This project fits the following aspects of ESD: fiber production from renewable sources other than cotton (wood-based cellulose); recycling of cellulose from its blends with synthetic polymers, when their reuse is not feasible. We recommend this project for senior science students of, e.g., chemistry, engineering and pharmacy, because of its simplicity, safety and socioeconomic relevance.
Keywords: Education for sustainable development, Ionic liquids, Cellulose dissolution, Cellulose regeneration, Chemical recycling of polycotton
Cite this paper: Omar A. El Seoud, Nicolas Keppeler, Education for Sustainable Development: An Undergraduate Chemistry Project on Cellulose Dissolution, Regeneration, and Chemical Recycling of Polycotton, Journal of Laboratory Chemical Education, Vol. 8 No. 1, 2020, pp. 11-17. doi: 10.5923/j.jlce.20200801.03.
Article Outline
1. Introduction
- Consumers prefer cellulose-based clothes especially in warm-, humid weather due to this biopolymer remarkable water (sweat) absorption capacity that is not matched by synthetic fibers [1]. Because of the increased world population, production of cotton- a water-intensive, sometimes pesticide intensive crop- is not expected to meet demand, leading to the so-called “cellulosic fiber gap” [2]. At present, the economically viable solution for closing this gap is to increase the use of cellulose from wood. Unlike cotton, cellulose from the latter source cannot be processed into fibers directly. It should be first dissolved in a given solvent and then precipitated, i.e., regenerated in a suitable bath of a non-solvent (so-called wet spinning process) [3,4]. As shown in Figure 1A, cellulose is a semi-crystalline biopolymer, i.e., its structure has crystalline and amorphous regions. This is due to the intra- and intermolecular hydrogen bonding (H-bonding) of the anhydroglucose units (AGUs), as seen in Figure 1B. Therefore, cellulose dissolution requires disruption of these hydrogen bonds, as well as the hydrophobic interactions present, because cellulose has an amphiphilic character [5].
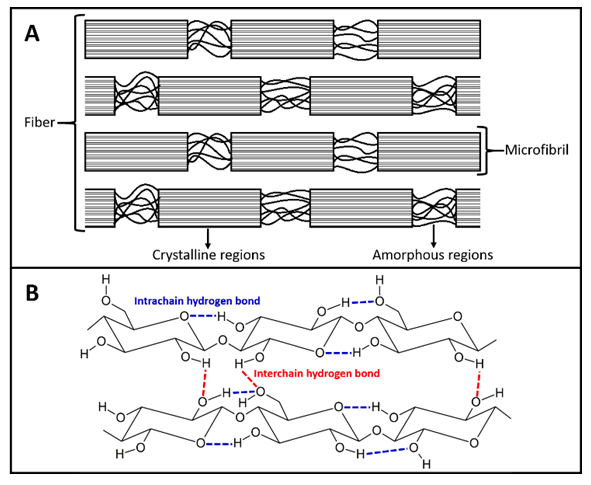 | Figure 1. A schematic representation of the semi-crystalline structure of cellulose (A), due to intra- and intermolecular hydrogen-bonding of the hydroxyl groups of the biopolymer AGUs (B) [7] |
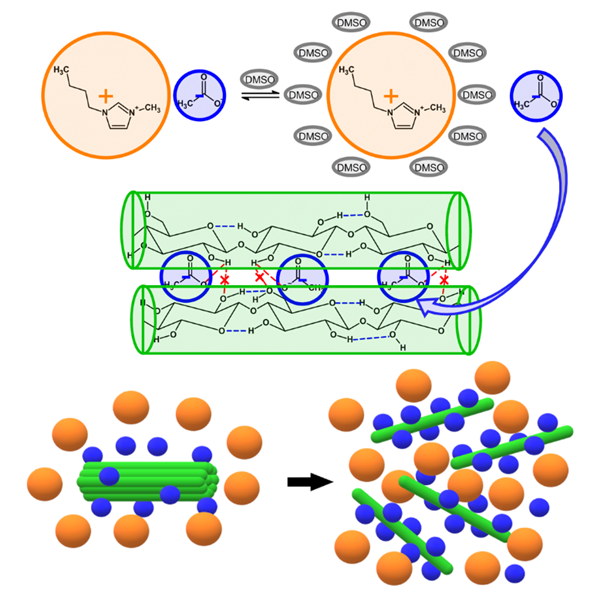 | Figure 2. Schematic representation of the hydrogen-bonding interactions of the ions of ILs with the hydroxyl groups of cellulose leading to its dissolution |
2. Experimental
2.1. Reagents and Polycotton
- The reagents (name and CAS number) 1-bromobutane (109-65-9) and 1-methylimidazole (616-47-7) were from Merck; ethyl acetate (141-78-6) and DMSO (67-68-5) were from Synth (São Paulo). The commercial activated montmorillonite clay was from Bentonisa (São Paulo). The polycotton sample (1:1 mixture of cellulose and PET) was supplied by Prof. Regina Sanchez of the textile department of the University of São Paulo. All substances and the textile blend were employed as received.
2.2. Equipment
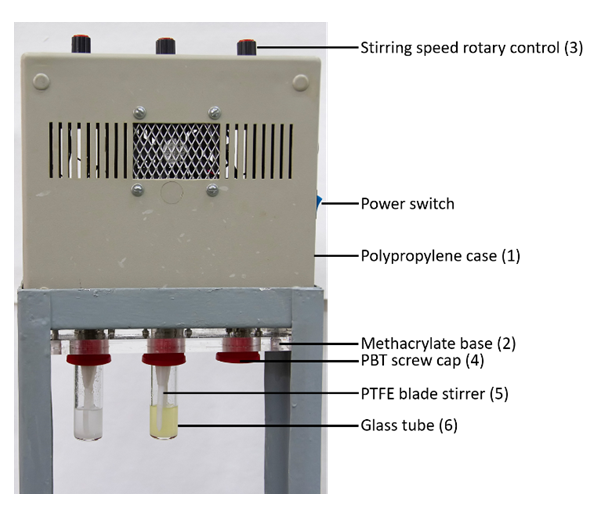
- The 1H NMR spectra were recorded by the instructor on Varian Inova model YH300 spectrometer (300 MHz for 1H). The students manipulated the provided free-induction decay (FID) signal using the academic edition of ACD/NMR processor [13]. IR analysis of the PET component of polycotton was carried out by the instructor using Bruker Vector-22 FTIR spectrophotometer; 1% PET/KBr pellet was used.The students dissolved MCC and the cellulosic component of polycotton using a home-built equipment described elsewhere [14]; see scheme in Figure 3.
2.3. Synthesis of 1-(n-Butyl)-3-methylimidazolium Bromide (BuMeImBr) and Its Transformation into 1-(n-Butyl)-3-methylimidazolium Acetate by Ion Exchange
2.3.1. Synthesis of BuMeImBr
- The following chemicals were added successively to 125 mL three-necked flask, provided with a reflux condenser protected with a drying tube (Drierite with indicator) and a magnetic stirring bar: 30 mL ethyl acetate (EtOAc); 17.0 mL 1-bromobutane (0.158 mol); 13.2 mL 1-methylimidazole (0.165 mol). The latter reagent was added slowly (5 minutes) to the 1-bromobutane/EtOAc solution, with efficient stirring at room temperature using 25 mL addition funnel. After completion of the addition, the mixture was stirred under reflux (diethylene glycol heating bath); total addition and reflux time = 2h. The reaction flask was cooled in an ice bath, and the mixture transferred to a separation funnel. The students carefully collected the lower phase (IL) into 125 mL Erlenmeyer flask, containing 30 mL of EtOAc, and agitated the suspension vigorously at room temperature. The students removed a sample of the upper layer, added it to water, agitated (vortex) and measured the pH of the aqueous (lower) phase; the latter was < 7 after two such washings. The students transferred the suspension to 125 mL separation funnel, carefully collected the lower phase into 50 mL Falcon tube and gave it to the instructor. The latter dried the IL under reduced pressure, over P4O10 until constant mass, and sent the result to the students to calculate the synthesis yield. The combined ethyl acetate (upper phase) was recovered by distillation; other residues were discarded by the safety division of this Institute.
2.3.2. Transformation of the Ion Exchange Resin from the Hydroxide form (Resin-OH) into Resin-AcO
- During the synthesis of BuMeImBr, the students transformed Resin-OH (Amberlite IRN 78, 1.20 equiv. OH-·mL-1) into the corresponding Resin-AcO, as follows: they slowly added 13.7 mL acetic acid (0.24 mol) to 200 mL of the resin-OH suspended in 500 mL of cold water (1 L Erlenmeyer flask). The students agitated the resin for 1 h and filtered it using a Büchner funnel. The students then suspended the resulting resin in water, agitated the suspension for 10 min, and measured the pH of the aqueous phase; the latter was ca. 5.5 after two washings. They gave the air-dried resin to the instructor. The water used for washing was neutralized with sodium bicarbonate and then discarded.
2.3.3. Transformation of BuMeImBr into BuMeImAcO by Ion Exchange
- The instructor performed this ion exchange, by pouring the Resin-AcO prepared by the students (200 mL) into a ground-joint Erlenmeyer flask. After covering the resin with methanol, he stoppered the flask, agitated the resin for 30 minutes at room temperature with a magnetic bar, and then transferred the suspension to a glass column with a sintered glass disc (length = 150 cm; od = 2 cm). He dissolved BuMeImBr (31.159 g, 0.142 mol) in 500 mL methanol, eluted the alcoholic solution slowly through the Resin-AcO column, followed by 200 mL of pure methanol.He tested the completeness of the (Br- → AcO-) anion exchange by treating a small aliquot of the eluent with aqueous AgNO3/HNO3; no precipitate was observed. He removed and redistilled the alcohol for future use. A yellowish color IL, 26.615 g (yield = 95 %) was obtained that gave the expected 1H NMR spectrum, vide section 3.3.
2.3.4. Dissolution of MCC in the Binary Mixture of BuMeImAcO-DMSO
- The students prepared the binary solvent mixture (by mass) with mole fraction χDMSO = 0.6. To the glass tubes (item (6) in Figure 3) they added 4 g pure DMSO and BuMeImAcO-DMSO. To each tube they suspended 160 mg of MCC (total cellulose content = 4 wt%) with aid of a vortex mixer to prevent lump formation. They assembled the tubes, as shown in Figure 3, and put them into a water bath whose temperature was previously adjusted (by the instructor) to 80°C (Aumax digital thermometer with Pt-100 probe, model N1540, São Paulo). The water surface in the bath was covered with polystyrene spheres to suppress water evaporation. The suspension was agitated at 150 rpm for 2 h. All MCC dissolved in BuMeImAcO-DMSO, rendering a yellowish solution. There was no visual change in the tube containing pure DMSO as solvent, see Figure 4A.The students employed a similar procedure for recycling of the cellulosic component of polycotton. To 4 g of pure DMSO and BuMeImAcO-DMSO they added 320 mg of polycotton (total cellulose content = 4 wt%) that was cut into 2 x 6 cm pieces. The fabric was agitated at 100 rpm for 2 hours, at 80°C. The tubes containing the fabric/solvent were then disassembled (see Figure 4B).
2.3.5. Regeneration of the Cellulose Dissolved in BuMeImAcO-DMSO
- For facilitating the detection of the regenerated fiber, and for a beautiful aesthetic aspect, the students dyed the dissolved cellulose by adding one drop of the super-base 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), followed by ca. 2 mg of the reactive dye (Procion® Red MX-5B), and agitation at room temperature for 10 minutes. An aliquot of the deep pink solution was withdrawn with 3 mL plastic disposable syringe, the latter was introduced into the holder of a syringe injection pump (SAGE Instruments, model 341B); the needle was inserted into a thin PTFE tube; the latter was inserted below the surface of water (150 mL) in a beaker, see Figure 5A. This arrangement made it easy to see the flow of the colored cellulose solution and biopolymer fiber regeneration, as shown in Figure 5B for regeneration under water interface, and Figure 5C for the fiber deposited on a glass rod.
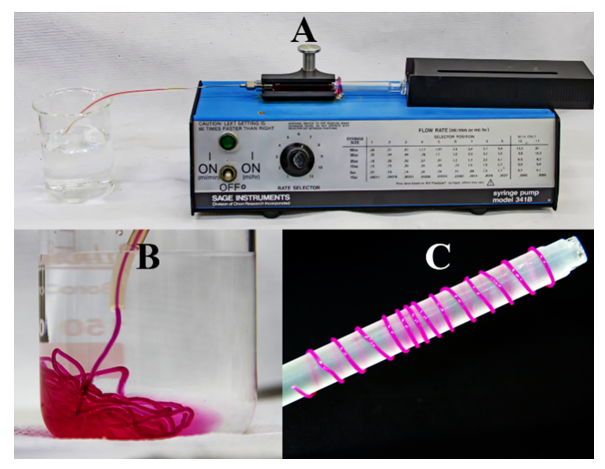 | Figure 5. Assembly of syringe injection pump (A), cellulose fiber regeneration under water (B), and cellulose fiber deposited on a glass rod (C) |
2.4. Hazards
- The students should perform the synthesis of the IL in a fume hood, using safety gloves and eye protection. 1-Bromobutane may cause respiratory irritation and 1-methylimidazole can cause skin burns and eye damage. EtOAc and 1-bromobutane are flammable and should be kept away from heat sources. Because of its negligible vapor pressure, the IL can be handled outside the fume hood.
3. Results and Discussion
3.1. Sustainability Context of the Project
- In response to the expected cellulosic fiber gap, the search for efficient, environmentally acceptable solvents to dissolve wood-based cellulose has been intensified [15,16]. Additionally, recycling of cellulose, e.g., from wastepaper, post-industrial waste, resulting from garment manufacturing, and post-consumer use (if re-use is not feasible) are also becoming important issues [17,18]. ILs, salts of super-bases, e.g., the carboxylates of DBU, and the structurally related 1,5-diazabicyclo[4.3.0]non-5-ene (DBN), and tetra(n-butyl)ammonium carboxylates are being investigated as efficient cellulose solvents with potential industrial application [19-23]. Use of ILs is advantageous, inter alia, because of their efficiency, and negligible vapor pressure, chemical and thermal stability, and possibility of recycling [24,25]. Thus, this project fits nicely into the objectives of ESD. The latter was declared by the United Nations in order to “integrate the principles, values, and practices of sustainable development into all aspects of education and learning, in order to address the social, economic, cultural, and environmental problems we face in the 21st century” [26]. By doing the experiment, the student verified the efficiency of a typical, easily synthesized IL as cellulose solvent relative to the strongly dipolar aprotic solvent DMSO that causes extensive cellulose swelling (> 100 fiber wt% increase), but not dissolution [10]. The experiment on recycling of the cellulosic component of polycotton addresses an increasingly important environmental problem because landfill of textile waste is not allowed in many countries [27]. Additionally, burning of colored (dyed) textile waste generates secondary pollution (oxides of nitrogen and sulfur) [28].
3.2. Details of the Project
- Chem-1405 is an advanced experimental chemistry course. The number of the enrolled students was 36. We divided them into eight groups, each of four- or five students; they perform different experiments in a round-robin style. Two groups synthesized BuMeImBr simultaneously and transformed Resin-OH into Resin-AcO in a four-hour laboratory period. They gave the IL and the converted resin to the instructor who did the transformation (BuMeImBr → BuMeImAcO) and gave the student the IL-acetate. In the next, four-hour laboratory period, the same students did the dissolution experiments using the special agitation equipment that we developed. One group worked on the dissolution of MCC in pure DMSO and in BuMeImAcO- DMSO mixture, the other group did the recycling experiment, also using the same solvents. As the students ran the two experiments close to each other, all were able to see the biopolymer dissolution progress; we commented on this aspect, where appropriate. Cellulose dissolution was evident in case of MCC, but not in the CR experiment. The reason is that a mat similar to the original fabric was visible in both solvents (DMSO and IL-DMSO), except for a difference in color. Therefore, the students did the regeneration experiment on the liquids in both tubes of Figure 4B. We introduced dyeing with the reactive dye not only for aesthetic purpose but, more importantly, because this dye reacts only with the cellulosic component of polycotton. Therefore, regeneration of a pink-colored fiber from the CR experiment in BuMeImAcO-DMSO, but not from the DMSO counterpart, showed that the cellulosic component was dissolved by the IL.Use of the dissolution equipment is a convenience, not a necessity. We found that the same experiment could be run using a magnetic bar instead of a mechanical stirrer, except that the spinning of the magnetic bar should be continuously watched.
3.3. Reporting the Data
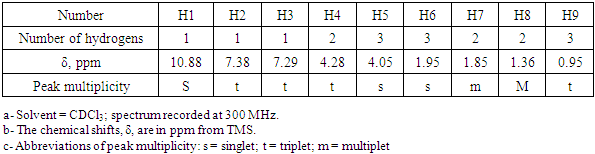
- During one hour in the classroom, the students reported the following data: - The yields of BuMeImBr (89%) and BuMeImAcO (95% for ion-exchange; final IL yield 84.5%); - Structure confirmation of BuMeImAcO. Figure 7 shows the 1H NMR spectrum of this IL in CDCl3, whereas the numbering of the corresponding hydrogen atoms is shown in Figure 8; the corresponding peak assignments are listed in Table 1. These results confirm the structure of the synthesized IL;- Recovery of PET from polycotton (ca. 55% yield; some undissolved cellulose remained);- Identification of the synthetic polymer by FTIR (ester νC=O at ca. 1730 cm-1) [29].
|
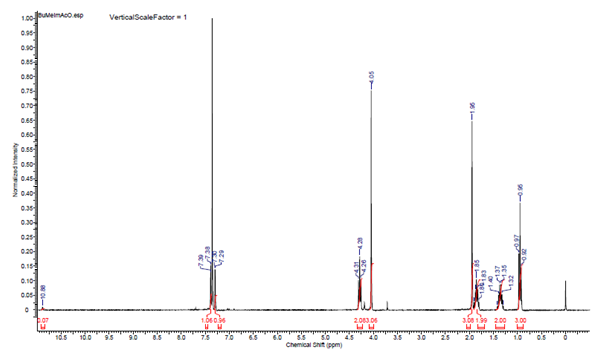 | Figure 7. 1H NMR spectrum of 1-butyl-3-methyl imidazolium acetate in CDCl3 |
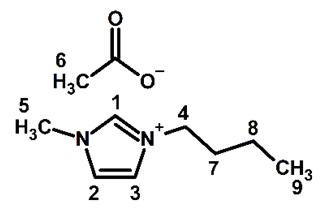 | Figure 8. The molecular structure of the synthesized BuMeImAcO with the numbering used for the assignment of the 1H NMR peaks of Table 1 |
4. Conclusions
- We introduced a project within the framework of ESD. The experiments are simple, safe, low-cost, and do not require sophisticated infrastructure. They highlight the need to address problems with important socioeconomic impact: developing efficient, environmentally acceptable solvents to dissolve cellulose from sources other than cotton for further processing, e.g., to produce fibers. The project shows one solution to the problem of recycling of post-industrial waste from the garment industry, and post-consumer waste, when re-use is not feasible. We recommend this project for senior undergraduate science students, e.g., those whose majors are chemistry, engineering, and pharmacy, because of the simplicity and safety of the experimental part, and the socioeconomic importance of the issues addressed.
ACKNOWLEDGEMENTS
- We are grateful to the students who participated in this project, Cezar Guizzo for help with photographing, and Prof. Regina Sanchez of the FFLCH-USP (São Paulo) for providing the polycotton sample. O. A. El Seoud thanks FAPESP (grant 2014/22136-4) and CNPq (grant 306108/2019-4) for financial support and for research productivity fellowship, respectively; N. Keppeler thanks CNPq for PhD fellowship (grant 141853/2019-0).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML