-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2020; 8(1): 6-10
doi:10.5923/j.jlce.20200801.02

Splitting Patterns in 1H NMR to Demonstrate a Reaction Mechanism
Jessica Epstein, Zanib Mian, Stephanie Rosales
Department of Chemistry, Saint Peter's University, Jersey City, USA
Correspondence to: Jessica Epstein, Department of Chemistry, Saint Peter's University, Jersey City, USA.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This experiment introduces second semester organic chemistry students to a deuterium transfer experiment to evaluate a reaction mechanism. Students gain direct experience preparing and running an NMR sample, experimentally confirm a reaction mechanism and interpret splitting patterns with 1H-NMR spectra. This experiment makes use of the reduction of a ketone, 3-hydroxy-acetaphenone, to an alcohol, 3-hydroxy-1-phenylethanol using sodium borohydride (NaBH4) or sodium borodeuteride (NaBD4). Each pair of students performs a reduction reaction with either protonated or deuterated conditions. Pairs of students then combine into groups of 4 to compare spectral data. Students observe the change in the splitting pattern caused by deuterium incorporation into the alcohol product and can confirm the reaction mechanism.
Keywords: NMR Spectroscopy, Reaction Mechanism, Organic Chemistry
Cite this paper: Jessica Epstein, Zanib Mian, Stephanie Rosales, Splitting Patterns in 1H NMR to Demonstrate a Reaction Mechanism, Journal of Laboratory Chemical Education, Vol. 8 No. 1, 2020, pp. 6-10. doi: 10.5923/j.jlce.20200801.02.
Article Outline
1. Introduction
- NMR spectroscopy is a fundamental skill for many chemical professions and is frequently taught at the undergraduate level by lecture, followed by worked examples and additional problems from the textbook [1-4]. There are also several tools to improve NMR interpretation skills using commercial software, guided inquiry, partial structure templating and other activities [5-8].While lecture can cover the theory of NMR, at some point it is important for students to connect NMR concepts taught in lecture with the usefulness of NMR in the organic chemistry laboratory for structure determination. Many organic chemistry synthesis experiments require an NMR (and IR) of the final product to confirm structure identity. In this process, students are usually asked to identify a solvent, prepare a sample and run a spectrum [9]. Historically, the hands-on undergraduate experience with NMR is limited to sample submission for NMR analysis. Multiple lab sections with 20+ students render it impractical and costly for inexperienced students to run samples on an instrument that requires the assistance of a trained technician. The advent of the teaching bench top NMR allows for new teaching opportunities at the undergraduate level [10-13]. A bench top instrument can be placed directly in the organic chemistry laboratory where the instructor can oversee both the proper use of the instrument as well as the activities of all the students at various stages of an experiment. There are three isotopes of hydrogen that can potentially be used for NMR: 1Hydrogen, 2Deuterium and 3Tritium. Each isotope resonates at a different frequency, and only one isotope can be observed at a time. Tritium is seldom used because it is radioactive. Deuterium NMR and proton NMR require very different operating frequencies. Therefore, deuterium is not detected under the operating conditions for proton NMR. This renders deuterium effectively ‘silent’ in proton NMR and explains why proton NMR samples are dissolved in deuterated solvents. A solvent with protons would completely overpower the absorptions of the sample. Deuterium can also serve as a potential research tool for any reaction that involves the transfer of hydrogen. There are numerous examples of research articles that make use of deuterium transfers experiments to explore reaction mechanisms, probe stereochemistry and explore reaction kinetics [14-16]. The technique has application in many specialties of chemistry including drug development and protein biochemistry [17-18]. However, it is usually not emphasized at the undergraduate level. There are a few teaching articles that utilize a deuterium transfer experiment with small organic molecules to introduce undergraduates to kinetics and stereochemistry. Acetaphenone has been deuterium labelled by reduction with NaBD4 followed by a study on the kinetic isotope effects using the alcohol product [19,20]. We found this experiment impractical at the sophomore level, because the product, a liquid at room temperature, is nearly all lost after the final work-up and drying. In addition, pure product for NMR requires sufficient material for distillation of the liquid product, and the reaction is costly to scale up due to the expense of NaBD4. Other experiments explore the stereo-selectively of NaBH4 reduction using a pro-chiral ketone [21,22]. Most organic chemistry textbooks cover the reduction mechanism shown in Figure 1 [1-4] and the specifics of the mechanism have been previously explored in the teaching laboratory with the reduction of vanillin [23]. The drawback of the vanillin reduction is the primary alcohol product, with no neighbouring protons, does not generate a splitting pattern that is affected by deuterium incorporation.In this experiment, students perform a reduction of 3-hydroxyacetaphenone using either NaBH4 or NaBD4, compare the NMR spectra of the products, locate the hydrogen from the reducing agent on the product and explore how deuterium incorporation into the product changes the spin-spin splitting pattern of the product. The product, 3-hydroxy-1-phenyl ethanol, is a solid at room temperature making purification simple, quick and efficient, representing a significant improvement over similar experiments.
2. Experimental
2.1. Experimental Overview
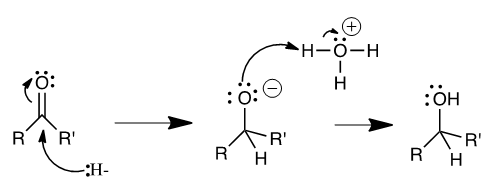
- NaBH4 is a reducing agent with a variety of applications. Although it is not as powerful as lithium aluminium hydride (LiAlH4), it is very effective and much safer for inexperience students to use for the reduction of ketones or aldehydes. Most organic chemistry students first encounter NaBH4 during their first semester in the second step of oxymercuration (conversion of an alkene to and alcohol). By itself, NaBH4 will not reduce esters, carboxylic acids or amides [1].The ketone 3-hydroxy-acetaphenone was chosen for the several reasons. The product is a solid at room temperature, making it easy for students to crystalize and isolate pure product. We found liquid products prohibitive, because they require a distillation step, which is impractical with exceedingly small volumes. The solid product yields are high, providing sufficient material for an NMR with a strong signal to background ratio. Finally, a ketone with protons on the neighbouring carbon makes for a more interesting splitting pattern to interpret, particularly if deuterium is incorporated and the splitting pattern changes. The reduction mechanism proceeds in two steps (Scheme 1): In the first step, hydride (:H-) detaches from BH4- and attacks the carbonyl carbon. Pi-electrons simultaneously move onto oxygen to form the alkoxide ion. In the second step, the non-bonding electrons on oxygen pick up a proton from solvent as the ion is neutralized with acid [1].
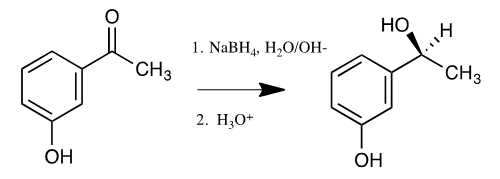 | Scheme 2. The reduction of 3-hydroxyacetaphenone with NaBH4 |
2.2. Experimental Procedure
- During the first week, 0.500g of 3-hydroxyacetaphenone is dissolved in 5.0 mL of 1.0 M NaOH. The mixture is warmed to completely dissolve the ketone and then cooled to room temperature (25°C) before starting the reaction. The reaction is started by adding 0.100 g of NaBH4 or NaBD4 and gently swirling the flask. To prevent contamination of the bottle, the instructor should pre-weigh NaBH4 or NaBD4. The reaction is complete after 1 hour and students can check the progress of the reaction by TLC at 30 minutes and again at 1 hour. Samples are spotted on silica TLC plates (Carolina Labs) and developed in 50% ethyl acetate and 50% hexane. Students are reminded to spot a sample of the starting material next to the reaction spot for comparison.After confirming that the reaction is complete, the flask is placed on an ice bath and 5 M HCl added drop wise, with continuous swirling (typically about 16-17 drops) until the reaction no longer bubbles. Bubbling and foaming are normal, however, students are cautioned that adding HCl too quickly results in a more violent reaction, bubbling over and subsequent loss of product. A disposable pH indicator strip (Carolina Labs) confirms that the solution is no longer basic and the flask is left on ice for an additional 20 minutes to allow crystals to form. Crystals are collected by vacuum filtration and washed with small (5-7 mL) portions of cold water. Crystals are allowed to air dry until the following week. The following week, students obtain the mass, melting point and IR (Nicolet FTIR Thermo Fischer) for their product. The instructor demonstrates how to prepare an NMR sample and students are directed to watch a video on the topic [24]. NMR samples are prepared in Acetone-d6, because the alcohol product is insoluble in most other solvents. Approximately 10-15 mg of product generates a strong signal to background ratio. NMR spectra are obtained on the Nanalysis-NMReady 60e. The instructor then pairs groups together so that each group of 4 students has one NMR spectrum of the protonated product and a second NMR spectrum of the deuterated product.
2.3. Safety Precautions
- Chemicals in this experiment are eye, skin and respiratory irritants. Safety goggles, laboratory coats and disposable gloves should be worn when working with these chemicals. In addition, the product, 3-hydroxy-1-phenyl ethanol, and acetone-d6 are flammable.
3. Results and Discussion
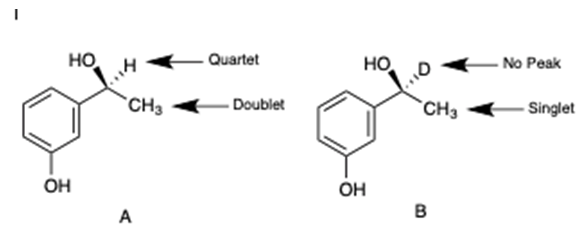
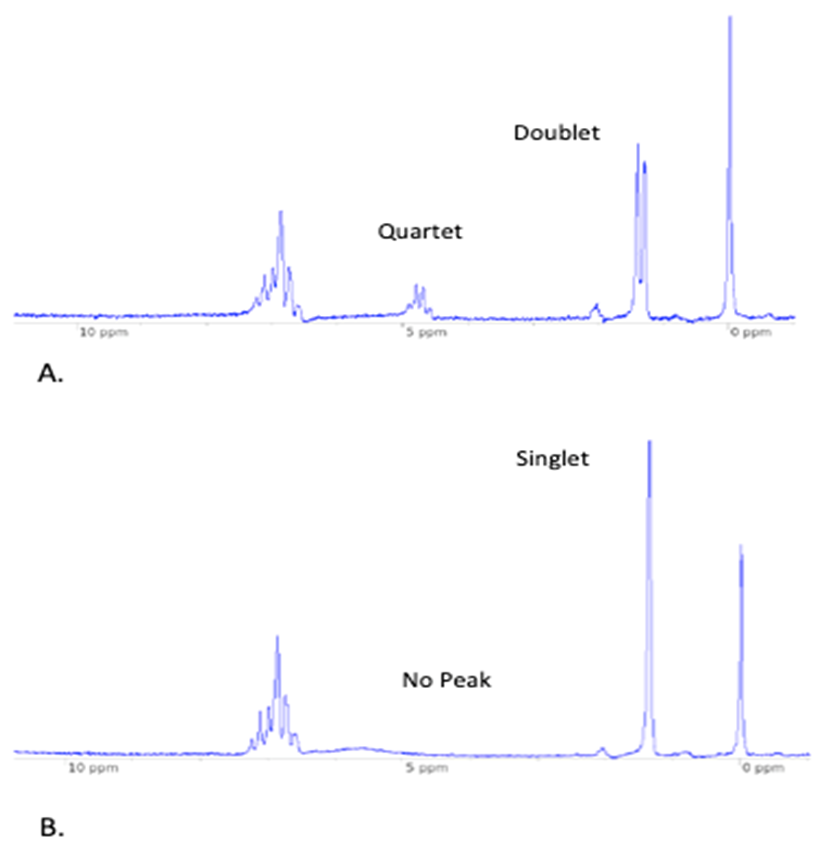
- As shown in Figure 2, when NaBH4 is used, the NMR spectrum of the product contains a quartet and doublet. When the reaction is carried out with NaBD4, the NMR spectrum of the product shows no signal for deuterium and the neighbouring methyl protons present as a singlet. The aromatic peaks remain the same in both sets of spectra. The disappearance of the peak at 4.81 ppm proves that hydride (or deuteride) originating from NaBH4 (or NaBD4) attacks the carbonyl as shown in Scheme 1. Since all other components of the experiment are protonated, the hydride must originate from NaBH4.
4. Conclusions
- This experiment gives second semester organic chemistry laboratory student’s hands-on experience with NMR. It reinforces concepts of chemical shift, spin-spin splitting and introduces deuterium transfer as a tool to deduce a reaction mechanism. The product, a solid at room temperature, represents a significant improvement over similar experiments, making it easy and economical for students to crystalize and isolate pure product for NMR analysis.
ACKNOWLEDGEMENTS
- The authors would like to thank Charles Vickers, Saint Peter's chemistry alumnus for his generous donation of the our teaching bench top NMR instrument and Michael J. Castaldi, Saint Peter's alumnus and retired instructor in the organic chemistry laboratory, for his helpful insight.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML