-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2020; 8(1): 1-5
doi:10.5923/j.jlce.20200801.01

A Physical Chemistry Escape Room Laboratory: Is Schrödinger’s Cat Dead and/or Alive?
Todd Wimpfheimer
Department of Chemistry and Physics, Salem State University, Salem MA, USA
Correspondence to: Todd Wimpfheimer, Department of Chemistry and Physics, Salem State University, Salem MA, USA.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Escape rooms have become increasingly popular and have recently been used to deliver mainly general chemistry laboratory experiences. This paper describes a laboratory experiment suitable for upper level students in physical chemistry that have had exposure to quantum chemistry. An escape room approach is used where students solve challenges and perform wet chemical manipulations in order to determine whether Schrödinger’s Cat is dead and/or alive.
Keywords: Physical chemistry, Quantum chemistry, Upper division undergraduate, Laboratory experiment
Cite this paper: Todd Wimpfheimer, A Physical Chemistry Escape Room Laboratory: Is Schrödinger’s Cat Dead and/or Alive?, Journal of Laboratory Chemical Education, Vol. 8 No. 1, 2020, pp. 1-5. doi: 10.5923/j.jlce.20200801.01.
Article Outline
1. Introduction
- Escape rooms, where people are “locked” in a room and must solve puzzles within a certain time frame in order to escape, have become increasingly popular. The concept may be transferred to a chemistry laboratory where wet chemical methods are incorporated to help solve puzzles or perform other challenges. Several authors have employed this technique with general chemistry students, either at the high school or freshman level or as outreach projects [1-3]. Others have featured experiments using instrumental methods [4], appropriate for upper class students. This paper describes a laboratory experiment appropriate for upper level students of physical chemistry, in whatever semester they study quantum chemistry. It makes use of advanced mathematics such as calculus as well as molecular modeling using PC Spartan software. The laboratory also includes several lab techniques usually not seen at the freshman level such as handling liquid nitrogen and utilizing the magnetic susceptibility balance. The students must solve a series of challenges taking them to a final locked box containing “Schrödinger’s Cat”. The Schrödinger’s Cat paradox is usually discussed in physical chemistry. The paradox, developed by Erwin Schrödinger, involves a cat postulated to be placed into a box along with a vial of a poisonous radioactive substance. The vial is potentially broken, but the cat is not known to be either dead or alive until the box is opened and observed. In some interpretations the cat is therefore both alive and dead until the box is opened. This paradox teaches the student, among other things, that in quantum chemistry in order to “see” the system, it is necessary for a photon of light to interact with the system. Quantum chemistry concerns bodies so small (eg. electrons) that this interaction could be enough to change its energy, position, momentum, etc. This paper presents a laboratory experiment that is intended to be a fun and interactive way for students to review lab techniques, quantum mechanical concepts and mathematical manipulations. By working together to solve problems under a time constraint and without direct instructions, it also helps students to practice “soft skills”: time management, collaboration, leadership, and teamwork.
2. Overview
- This laboratory experiment is intended to run late in the semester of the physical chemistry course that contains quantum chemistry. At Salem State University Physical Chemistry I is that course. The student population of the course is almost exclusively chemistry majors. The experiment is intended to last one hour, like many escape rooms, and is intended for student groups of 5-8. Also, like escape rooms, students are given access to three “hints” so that they don’t become frustrated. The setting is the chemical laboratory where lab has taken place all semester. Several lockboxes or other containers that can be locked are required. Multiple locks are also required. Some of the fun of escape rooms is encountering different types of locks that must be opened, so this experiment utilizes many kinds of locks such as numerical, alphabetical, key, and even locks that can be ignited and burned off.Students are met outside the laboratory and are briefed on the process. They are to solve “challenges” in an effort to find Schrodinger’s Cat and to determine if it is dead and/or alive. Some of the challenges are puzzles, some involve calculations, some involve review general chemistry laboratory techniques, and others are physical chemistry laboratory techniques. For this particular escape room style lab, there are four different starting points and many of the challenges can be undertaken simultaneously. Ultimately, four different experimental measurements must be determined in order to solve the code to unlock the final box. A diagram of how the challenges flow into one another is provided in Figure 1.
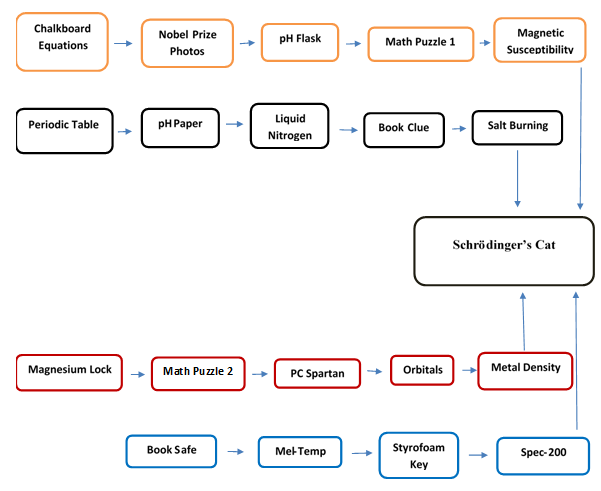 | Figure 1. Diagram of Challenge Flow |
3. Challenge Descriptions
- Chalkboard Equations – Written on the front chalkboard are incomplete equations:
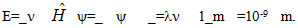 When completed, the letter combination opens a locked notebook containing the Nobel Prize Photos challenge.Nobel Prize Photos – This notebook contains a list of Nobel Prize winners in physics by year, along with photos of five of the winners with this clue from Erwin Schrödinger: “My colleagues won Nobel Prizes in Physics in various years. The Photoelectric Effect – Quanta + the Uncertainty Principle -The Exclusion Principle + my Cat”. Matching each winner with their year and performing the appropriate addition and subtraction gives the combination to open the locked box containing the pH Flask challenge.pH Flask [1] – This box contains a small Erlenmeyer flask containing NaOH(aq) made dark pink by the addition of phenolphthalein, along with the clue: “Lower the pH of the pink solution until the secret message is revealed”. On the bottom of the flask written in pink permanent marker is the secret message: “stir bars”. There are nearby bottles of water, HCl(aq), NaOH(aq). Addition of acid clears the solution and allows the message to be seen. Nearby there are stir bars that are hiding a key that opens a locked box containing Math Puzzle 1.
When completed, the letter combination opens a locked notebook containing the Nobel Prize Photos challenge.Nobel Prize Photos – This notebook contains a list of Nobel Prize winners in physics by year, along with photos of five of the winners with this clue from Erwin Schrödinger: “My colleagues won Nobel Prizes in Physics in various years. The Photoelectric Effect – Quanta + the Uncertainty Principle -The Exclusion Principle + my Cat”. Matching each winner with their year and performing the appropriate addition and subtraction gives the combination to open the locked box containing the pH Flask challenge.pH Flask [1] – This box contains a small Erlenmeyer flask containing NaOH(aq) made dark pink by the addition of phenolphthalein, along with the clue: “Lower the pH of the pink solution until the secret message is revealed”. On the bottom of the flask written in pink permanent marker is the secret message: “stir bars”. There are nearby bottles of water, HCl(aq), NaOH(aq). Addition of acid clears the solution and allows the message to be seen. Nearby there are stir bars that are hiding a key that opens a locked box containing Math Puzzle 1. | Figure 2. pH challenge |
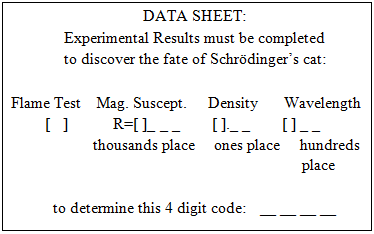
 The correct numerical answers open a nearby locked box containing the Magnetic Susceptibility challenge.Magnetic Susceptibility – This box contains a labeled sample of MnO and a clue: “Use the Magnetic Susceptibility Balance to measure the reading, R, of 0.7 cm of a tightly packed sample and record your data on the data sheet in the appropriate place.” Nearby there is a magnetic susceptibility balance and sample holders. Once the measurement is made, a digit of the final code can be recorded on the data sheet at the front of the room.Periodic Table – In plain sight on a lab bench is a periodic table, along with a piece of thin foam board with some strategically cut holes in it. Proper placement of this foam board overlaying the periodic table and following arrows written on the foam board yields four chemical element symbols which opens the letter lock containing the pH Paper challenge.
The correct numerical answers open a nearby locked box containing the Magnetic Susceptibility challenge.Magnetic Susceptibility – This box contains a labeled sample of MnO and a clue: “Use the Magnetic Susceptibility Balance to measure the reading, R, of 0.7 cm of a tightly packed sample and record your data on the data sheet in the appropriate place.” Nearby there is a magnetic susceptibility balance and sample holders. Once the measurement is made, a digit of the final code can be recorded on the data sheet at the front of the room.Periodic Table – In plain sight on a lab bench is a periodic table, along with a piece of thin foam board with some strategically cut holes in it. Proper placement of this foam board overlaying the periodic table and following arrows written on the foam board yields four chemical element symbols which opens the letter lock containing the pH Paper challenge.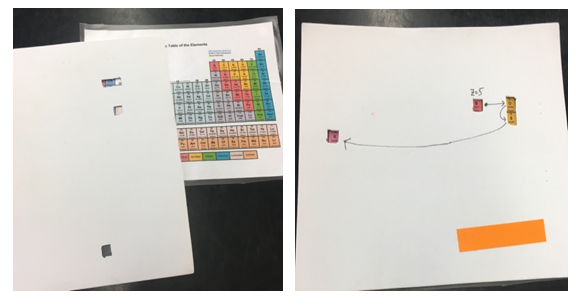 | Figure 3. Periodic table challenge |
 | Figure 4. Liquid nitrogen challenge |
 | Figure 5. Mg lock challenge |
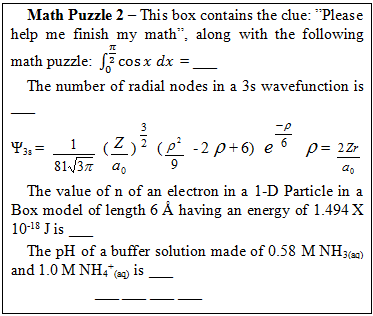 The correct numerical answers open a nearby locked box containing the PC Spartan challenge.PC Spartan – The box contains a molecular model of 1-bromopropane, a hasp needed to run our PC Spartan molecular modeling software, and the following clue:
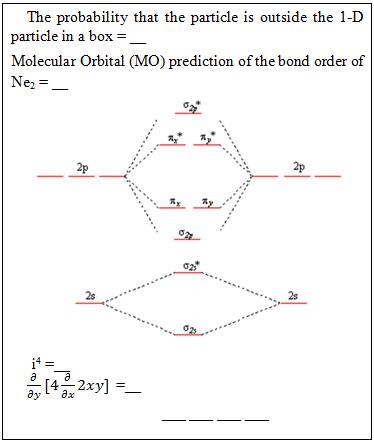
The correct numerical answers open a nearby locked box containing the PC Spartan challenge.PC Spartan – The box contains a molecular model of 1-bromopropane, a hasp needed to run our PC Spartan molecular modeling software, and the following clue: 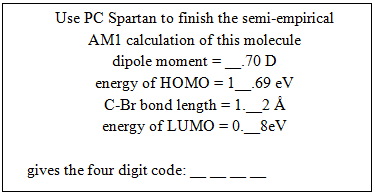 There is a nearby computer to run the PC Spartan software. The correct answers yield the numerical code to open the locked box containing the Orbitals challenge.Orbitals – The box contains the following clue, (orbital models are available on the lab benches for reference)
There is a nearby computer to run the PC Spartan software. The correct answers yield the numerical code to open the locked box containing the Orbitals challenge.Orbitals – The box contains the following clue, (orbital models are available on the lab benches for reference)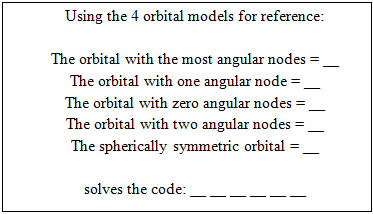 The correct answers yield the code that opens the letter lock to the box containing the Metal Density challenge.Metal Density – The box contains a sample of an unknown metal (aluminum) and the clue: “Measure the density in g/mL of this solid and record your data on the data sheet in the appropriate place.” Once the measurement is made, a digit of the final code can be recorded on the data sheet at the front of the room.Book Safe – Lying in plain sight on a lab bench is a chemistry book safe containing the clue: “I am the grandfather of Olivia Newton-John and the founder of the interpretation that the square of the normalized wavefunction yields the probability of finding the particle at that position” solves the code: __ __ __ __ There is also a labeled photo of Max Born and Olivia Newton-John in plain sight in the laboratory. Born’s name unlocks the letter lock to the box containing the Mel-Temp challenge.
The correct answers yield the code that opens the letter lock to the box containing the Metal Density challenge.Metal Density – The box contains a sample of an unknown metal (aluminum) and the clue: “Measure the density in g/mL of this solid and record your data on the data sheet in the appropriate place.” Once the measurement is made, a digit of the final code can be recorded on the data sheet at the front of the room.Book Safe – Lying in plain sight on a lab bench is a chemistry book safe containing the clue: “I am the grandfather of Olivia Newton-John and the founder of the interpretation that the square of the normalized wavefunction yields the probability of finding the particle at that position” solves the code: __ __ __ __ There is also a labeled photo of Max Born and Olivia Newton-John in plain sight in the laboratory. Born’s name unlocks the letter lock to the box containing the Mel-Temp challenge. | Figure 6. Book safe |
 | Figure 7. Mel-Temp challenge |
 The box is labeled with the following: “Schrödinger’s cat is inside; Give this box a good shake before opening.” The box contains a six-sided foam die; three sides show a cat that is alive, and three sides show a cat that is dead. Once the code from the experimental data sheet is determined, the students shake the box, unlock it and open it to determine whether Schrödinger’s cat is dead and/or alive.
The box is labeled with the following: “Schrödinger’s cat is inside; Give this box a good shake before opening.” The box contains a six-sided foam die; three sides show a cat that is alive, and three sides show a cat that is dead. Once the code from the experimental data sheet is determined, the students shake the box, unlock it and open it to determine whether Schrödinger’s cat is dead and/or alive.4. Hazards
- HCl(aq) and NaOH(aq) are corrosive. MnO and Cu(NO3)2(s) are irritants and should not be ingested. KMnO4(aq) is an oxidizing agent. Acetone and magnesium metal are flammable. Liquid nitrogen can cause frostbite or severe cryogenic burns. Sr(NO3)2(s) is an oxidizer and irritant and should not be ingested. Students should wear protective eyewear during the laboratory.
5. Discussion
- This laboratory experiment has been run seven times. It is unlike a usual laboratory experiment in that many students are in different parts of the lab doing different things, so it is fairly challenging to proctor. Our laboratory period is 3 hours long with an enrollment of 10-14 students per lab section. These students were divided into two groups of 5-7 with one group doing the lab for the first hour of the period. There is then 30-60 minutes allowed to set the lab back up again and the second group can then take the last hour of the period. This escape room lab experiment was a fun and rewarding way to review chemistry concepts and techniques. Having groups of approximately 6 students complete 20 challenges in under an hour requires the students to collaborate, share results, and manage time. During the experiment the lab is full of energy with students working together, comparing approaches, and troubleshooting. The puzzles and challenges are presented here for easy adoption. They have worked very well with the favorite ones being the burning of the magnesium lock, the liquid nitrogen, and the Nobel Prize photo challenge. The participants completed a post-lab survey asking them to rate their opinion of the lab on a scale of 1(poor)-5(excellent), with the average being a 4.7. Anecdotally, about 20% of all the students that have run this experiment have expressed that it is their favorite lab that they have ever done! In conclusion, this lab experiment has been a fun, positive, interactive way for upper level chemistry majors to review physical chemistry concepts.
ACKNOWLEDGEMENTS
- The author thanks Leah Wimpfheimer for helpful discussions.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML