-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2019; 7(2): 23-32
doi:10.5923/j.jlce.20190702.01

Combined Use of Macro and Microscale Chemistry in Undergraduate Organic Laboratory
Laila Laasri, Saïd Sebti
Chemistry Department, Faculté des Sciences Ben M'Sik, Hassan II University of Casablanca, Laboratoire de Chimie Physique Catalyse et Environnement (LCPCE), Casablanca, Morocco
Correspondence to: Laila Laasri, Chemistry Department, Faculté des Sciences Ben M'Sik, Hassan II University of Casablanca, Laboratoire de Chimie Physique Catalyse et Environnement (LCPCE), Casablanca, Morocco.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The current study represents a pilot experiment conducted at University Hassan II of Casablanca, which aims to introduce gradually a new practical teaching approach combining macro and microchemistry into organic chemistry curriculum for the third-year undergraduate chemistry students. A set of fast and simple experiments were introduced over two phases, a simple level of practical instruction in the functional organic chemistry course taught in semester 5 and an advanced level in the practical teaching of green chemistry course taught in semester 6. We used both macro and microscale laboratory techniques and equipment which allowed us to use fewer reagents and organic solvents and to enhance students' skills in mastering macro and micro techniques often used in industrial and research laboratories. Several green metrics as well as Green Star metric were calculated to evaluate the greenness of the implemented experiments. It appeared from the results that aldol, phospho-aldol and Knoevenagel condensations gives a very interesting environmental performance and can be considered as an excellent example of green experiments for undergraduate organic chemistry labs. The survey conducted among third-year chemistry students showed that 84% of the students agreed that introducing macro and microscale chemistry approach has enhanced their interest in learning organic chemistry.
Keywords: Macro and micro-scale organic experiments, Third-year undergraduate students, Green metrics, Students' perception
Cite this paper: Laila Laasri, Saïd Sebti, Combined Use of Macro and Microscale Chemistry in Undergraduate Organic Laboratory, Journal of Laboratory Chemical Education, Vol. 7 No. 2, 2019, pp. 23-32. doi: 10.5923/j.jlce.20190702.01.
Article Outline
1. Introduction
- Organic chemistry is a part of science that has a large application in everyday lives, the mastery of the basic concepts and techniques of organic chemistry can lead improving competence in many other fields 1. At the same time and according to many publications, organic chemistry is perceived as a challenging and difficult undergraduate science course. Students prefer to resort to memorization in order to pass their exams and they are unable to reason 2-4.Practical work is one of the effective ways to enhance students' knowledge and skills and the laboratory work has a rightful place in undergraduate courses, it helps students to get better understanding especially when the aim of the experimentations is clearly defined 5. However, considerable time and money are spent on practical activities, this may limit its development in institutions with a financial shortfall 6. In this case, microscale chemistry seems to be an adequate way to solve this problem.Indeed, microchemistry has been very well developed in high schools due to the efforts made by the National Microscale Chemistry Center (NMCC) in Boston which has released this concept with the support of the Environment Protection Agency (EPA) and has been able to generalize microchemistry to high schools in the United States. In other parts of the world, microscale chemistry is becoming increasingly prevalent in the curriculum. Recently, in India, a manual of the microscale chemistry laboratory was developed based on the national curriculum framework, highlighted the use of microscale chemistry techniques in schools 7.At universities, despite the efforts made by some professors to integrate microchemistry experiments in practicals 8-11, there is very slow progress in setting-up this approach, according to Bradley 12 several factors are involved, among them, insufficient government support, resistance to change, non-adaptation to one's own context, etc. Additionally, Breuer has raised in his study the reasons why some people develop a resistance to the adoption of microscale chemistry, we quote, the low skillfulness of students, they don't meet standard equipment, the price of specific equipment required, the inadequacy with industry techniques and the negligence of safety on this scale 13.To solve these problems, the use of a new approach that combines micro and macrochemistry seems to be interesting. For this reason, we developed several experiment labs that mutually use macro and micro scale chemistry approaches. Our objective is to reduce the consumption of reagents and organic solvents, to work in a cleaner and more secure conditions and to allow students to become familiar with laboratory equipment and practices used in both industrial and research laboratories, especially that industrial analysis and control laboratories use small quantities to carry out their tests. Thereby, students should master both macro and micro techniques.We evaluated the greenness of the developed experiments and assessed the students’ perception regarding the relevance of introducing microchemistry in their learning process.
2. Experimental Section
2.1. General Procedures
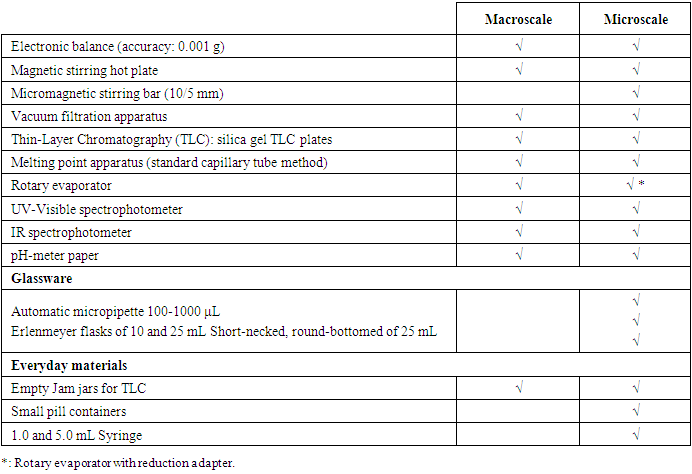
- Two sets of experiments were designed and implemented. The first one includes three experimentations whose main objective is to learn about aldol condensation, saponification and haloform reaction with a focus on the reaction mechanism studied in functional organic chemistry classes in semester 5. The second one is focused on the ecological aspect by applying the principles of green chemistry previously seen in green chemistry course thought in semester 6, specifically catalysis.For all experiments, the reaction medium was conducted at the microscale, the rest of the operations were performed using conventional macroscale equipment and techniques.0.1 to 1 gram of starting material were used. The reactions were carried out in small-scale glassware included conical flask of 10 mL or small pill container. Liquid reagents were often measured using 100 - 1000 μL automatic micropipette or syringe with capacities ranging from 1 to 5 mL, and masses were measured using electronic balance with precise accuracy of 0.001 g.For working up the reaction, classical equipment usually required in macroscale experiments were used. The product isolation operations and characterization were performed using conventional macroscale laboratory analysis techniques.For the first set of experiments, the reaction products were solids and were collected by filtration using macroscale vacuum filtration apparatus and then washed with an appropriate solvent.The recrystallization process was used to purify the obtained crude products (experience 1 and 3). During the purification steps, we used the smallest glassware generally available in a traditional organic chemistry laboratory and a classic laboratory hot plate.For experiment 4 and 5, thin-layer chromatography (TLC) was used to monitor the progress of reactions. We used the classic system with silica gel TLC plate immersed in the eluting solvent previously transferred into an Empty Jam jar. A small spot of products was sufficient to conduct the test.The product of a reaction does not crystallize from the reaction mixture and was isolated by macroscale simple distillation using Rotary evaporator. We used a reduction adapter, to avoid transferring the reaction mixture to a larger container (evaporation flask) and therefore prevent the loss of the product.To verify the purity of the product, we conducted a melting point analysis using a standard capillary melting point apparatus requiring only a few crystals trapped in the capillary tube to perform the analysis.”Once the purity has been verified, structure determination was accomplished by using UV/Vis and IR spectroscopies methods since they require a very small amount of product to carry out the tests. Table 1 presents the required material for the experiments and shows that a large part can be used on both scales.
|
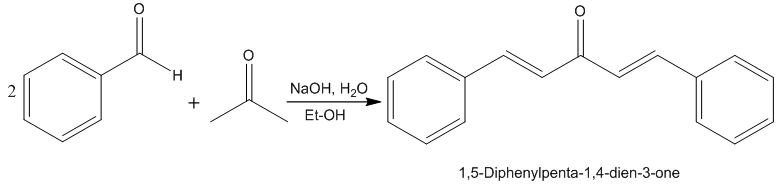 | Scheme 1. Reaction Scheme of DBA synthesis |
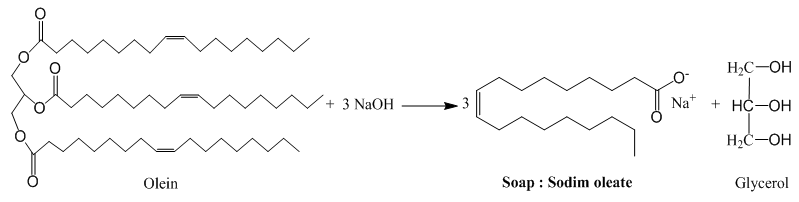 | Scheme 2. Saponification of olein |
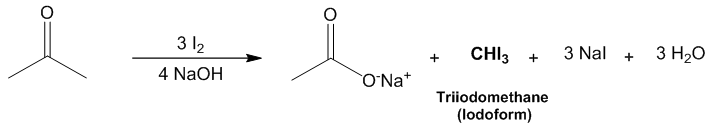 | Scheme 3. Reaction Scheme of triiodomethane synthesis |
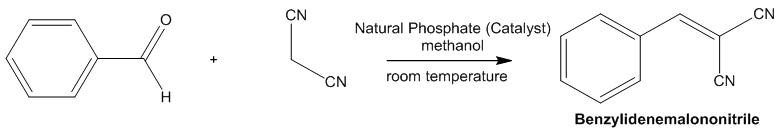 | Scheme 4. Reaction Scheme of benzylidenemalononitrile synthesis |
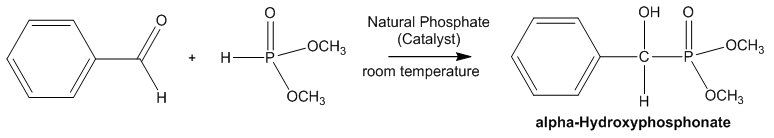 | Scheme 5. Reaction Scheme of α-Hydroxyphosphonate synthesis |
3. Results and Discussion
3.1. Reactions Yield
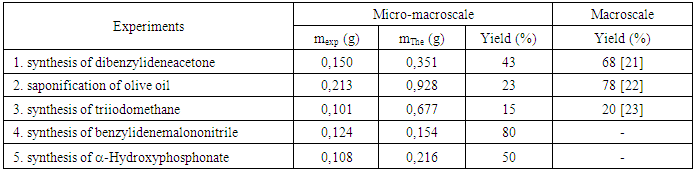
- Through the results obtained in table 2, we noticed that despite the low quantities used of the starting materials, the products are obtained in reasonable quantities. However, a decrease in yield was observed due to the reduced scale. The rate of this decrease depends on the experimental protocol followed in each experiment.
|
3.2. Environmental Assessment
- Carbon Efficiency, Atom Economy, Reaction Mass Efficiency and E-Factor are the parameters used to compare the environmental performance of the developed experiments. The Green Star metric was also considered to assess the benignness of experiments by considering simultaneously all of the principles of green chemistry.
3.2.1. Carbon Efficiency
- The carbon efficiency (CE) is calculated as the weight ratio of the number of carbon atoms of the product to that of the reactants (equation 1).
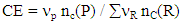 | (1) |
3.2.2. Atom Economy
- Trost stated in his article, that the Atom Economy (AE) of a chemical reaction measures the number of atoms in the reactants that end up in the final product 24.
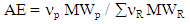 | (2) |
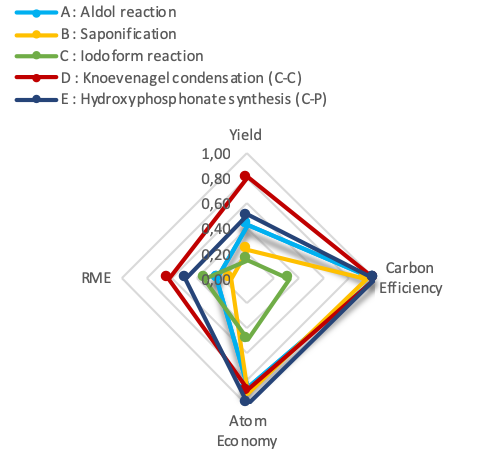 | Figure 1. Green metrics for the implemented experiments |
3.2.3. Reaction Mass Efficiency
- Reaction Mass Efficiency (RME) was mentioned for the first time by Clark in 1999 25, to describe green practices. The general expression for reaction mass efficiency (RME) is given by equation 3.
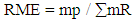 | (3) |
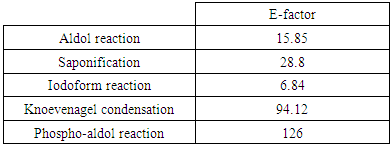
3.2.4. E-Factor
- Sheldon proposed the E-Factor (E) parameter in the late 1980s 26 and considered it as the most and popular parameter used to assess the environmental impact of manufacturing processes and to illustrate the problem of waste in different segments of the chemical industry. It can be calculated as illustrated in equation 4.
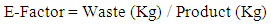 | (4) |
|
3.2.5. Green Star Metric
- The Green Star is a holistic metrics graphic developed by Ribeiro et al., 28 for evaluation of the global greenness of chemical reactions used in teaching laboratories by evaluating the accomplishment of each of the 12 principles of green chemistry.The construction of the graphical representation of GS was described in detail by Ribeiro et al., 29. First, we made an inventory of all the substances involved in each experiment: reactants, products, byproducts, solvents, catalyst, etc. We thus assign scores of 1 to 3 to each substance according to the information on the hazards to human health and the environment, the potential chemical accidents for each substance, as well as renewability and tendency to break down into innocuous degradation products (Table S1).The needed information’s were collected from safety data sheets (SDS) obtained in WEB (Sigma Aldrich and Merck Chemicals). The Criteria and scores to construct the GS are described by Ribeiro et al., 29.A Green Star Area Index (GSAI) is calculated as a percentage of green area of the green star/area of the green star of maximum greenness 29. Its values vary from 0 (minimum greenness) to 100 (maximum greenness).For each experiment, the GS was constructed, the results are presented in figure 2.
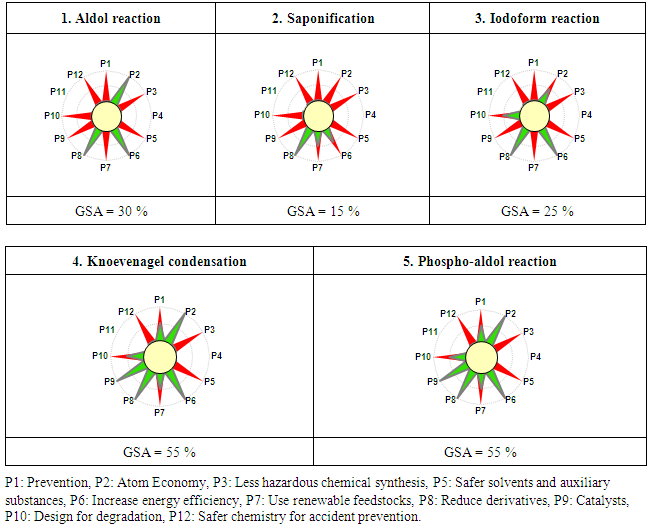 | Figure 2. Green Stars of the developed experiments |
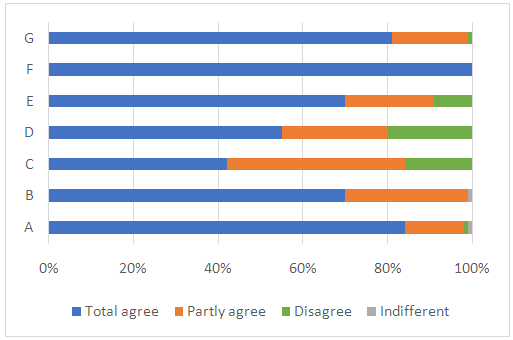
3.3. Student’s Perception toward Microscale Organic Chemistry Experimentations
- 79 students having followed macro and microscale experiments, filled the questionnaire at the end of the practical sessions. The students’ responses are presented in figure 3.
3.4. Specific Laboratory Handling Rules
- In addition to the classical rules commonly used in an organic chemistry laboratory, specific practices are to be considered.Material Cleanliness: The smooth running of the reaction is conditioned by a very clean material. The presence of impurities can reduce the percent yield and sometimes inhibits the reaction. The glassware should be rinsed 3 times with deionized water and then rinsed with acetone and dried in an oven, excepting volumetric material, we let them air dry.Weighing solids: Glass weighing boat is highly recommended. We should never use filter paper as containers, the weighed material will stick with the fibers of the filter paper which will lead to a significant loss of mass.Handling liquids: Automatic micropipette is the key equipment for successful experiments, it allows the sampling and the transfer of solutions. Syringe can also be used for transferring solutions to the reaction flask. These equipments should be kept clean after every use to avoid any possible contamination.
3.5. Safety and Waste
- We did not raise any particular safety precautions to be taken into consideration. Students should wear standard safety equipment, such as laboratory coat, safety glasses, and gloves.Solvent wastes were collected in a non-halogenated organic solvents waste container, except for the dichloromethane which was transferred into a container for halogenated organic solvents.
4. Conclusions
- The developed experiments are easy to implement and require equipment often available in organic chemistry labs with very low consumption of chemicals. Through the use of small amounts of chemicals, the reactions proceed quickly and generate less waste which is beneficial both financially and environmentally.Significant improvements can be made in almost all undergraduate organic chemistry practical class which require a large quantity of very often dangerous organic solvents and a large amount of reagents. By adopting this teaching strategy, the laboratory becomes less consumer of chemicals, cleaner, more secure and less pollutants while ensuring students' better adherence to the practical learning process.To compare the efficiency of the experiments, several green metrics are calculated. It appears from the results that aldol reaction, Knoevenagel condensation, and phospho-aldol reaction showed the best environmental profile, while, haloform reaction and saponification showed the lowest environmental performance because of byproducts. Thus, the mutual use of macro and microscale chemistry in the same experiment allows not only the achievement of educational objectives in convenient conditions, but it also brings students closer to micro and macro techniques usually practiced in both industrial and research laboratories. Through this work, we showed that it is possible to benefit from the advantages of mixed approaches in the same experiment.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML