-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2019; 7(1): 19-21
doi:10.5923/j.jlce.20190701.03

A Guided-Inquiry Experiment: Separation of a Five-Component Mixture by Liquid-Liquid Extraction and Column Chromatography
Ronald Okoth, Justin Keener, Myah Lanier, Peter Rosado-Flores
Department of Chemistry, Physics and Astronomy, Georgia College & State University, Milledgeville, USA
Correspondence to: Ronald Okoth, Department of Chemistry, Physics and Astronomy, Georgia College & State University, Milledgeville, USA.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this article we present a guided-inquiry based approach to separation of a five-component mixture using acid-base chemistry and column chromatography. In this approach students are required to come up with a viable protocol to successfully separate a five-component mixture of unknown compounds and use 1H NMR spectroscopy and melting point determination to identify the compounds. In guided-inquiry based approach presented here the students are assigned an unknown mixture containing a strongly acidic, weakly acidic, basic, polar-neutral and nonpolar-neutral components to separate and solutions to be used for extraction and the students are asked to draw a flow-chart outlining their separation protocol. This approach challenges the students to apply fundamental knowledge gained in lecture of acid-base chemistry and intermolecular forces to come up with a separation protocol.
Keywords: Guided-inquiry based liquid-liquid extraction, Acid-base extraction/column chromatography, Organic chemistry separation laboratory experiment
Cite this paper: Ronald Okoth, Justin Keener, Myah Lanier, Peter Rosado-Flores, A Guided-Inquiry Experiment: Separation of a Five-Component Mixture by Liquid-Liquid Extraction and Column Chromatography, Journal of Laboratory Chemical Education, Vol. 7 No. 1, 2019, pp. 19-21. doi: 10.5923/j.jlce.20190701.03.
1. Introduction
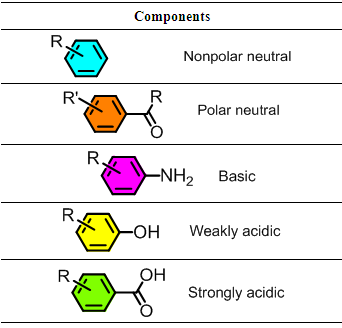
- Separation is one the fundamental techniques used for purification and isolation of compounds in the laboratory. Most separation techniques used in synthetic organic chemistry laboratories rely on acid-base chemistry and intramolecular and intermolecular forces. Acid-base chemistry allow for separation of mixtures via liquid-liquid extraction techniques. Intramolecular forces allow for separation of mixtures via distillation techniques and intermolecular forces allow for separation of mixtures via chromatography techniques and recrystallization techniques. The understanding of the fundamental principles behind these techniques is therefore crucial in training of organic chemists. In the late 1980s a laboratory experiment on separation of a five-component mixture was reported. This experiment was designed as “recipe-based” approach in which students follow a given procedure to achieve the separation. [1] Most recently, “recipe-based” approaches have been developed for a laboratory experiment on the separation of the components of a commercial analgesic tablet [2], and separation of two mixtures of unknown organic compounds [3]. Also, a guided-inquiry experiment in which students were assigned a mixture of compounds and are required to design their own separation procedures has been reported even though the mixture was of only three-components. [4]The guided-inquiry separation and characterization of the five-components in the unknown mixtures we report here will take a total of three laboratory meetings. In the first meeting the students are assigned unknown mixture containing a nonpolar-neutral component, a polar-neutral component, a basic component, a weakly acidic component and a strongly acidic component. Representative structures of compounds in the unknown mixtures are shown in table 1.Additionally, the students are provided with a list of reagents that they will use to perform the separation. The students are then required to design a separation protocol and draw a flow-chart outlining how the separation will be achieved.The acidic nature of the strongly acidic (benzoic acid derivatives, pKa ≈ 4) and the weakly acidic (phenol derivatives, pKa ≈ 10) components will allow their separation from the mixture and from each other. Similarly, the basic nature of the basic component (aniline derivatives) will allow for its separation from the mixture. The neural components will be separated from each other via column chromatography based on their polarity. Each component in the mixture will then be identified based on its characteristic 1H NMR spectra and melting point.
|
2. Method
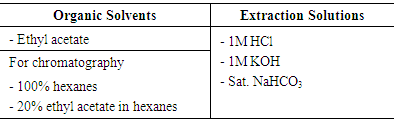
- Experimental ProcedureIn this experiment students may work in pairs or individually. On the first laboratory meeting students are assigned an unknown mixture containing the five components. Also, students are provided with solutions and solvents to be used in the experiment (table 2). Students are then required to design a procedure in the form of a flow chart (see example illustrated in figure 1) outlining how they intend to achieve separation of the mixture. The instructor has to approve the student’s suggested procedure before they can proceed with the experiment.
|
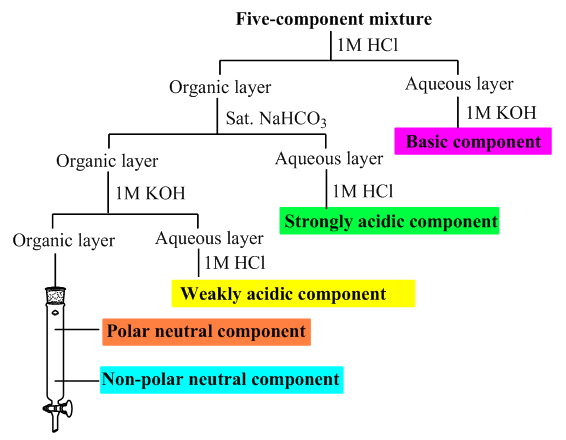 | Figure 1. An example of a flow chart outlining the separation procedure for a five-component mixture |
3. Results
- Most preliminary procedures were approved by the instructor of record on the first day of the experiment with minimal changes. Students were also asked to provide references to any source that they might have used for their work in proper ACS format.The majority of instructor changes to student led procedures included: wrong order of addition of reagents, wrong glassware usage, and wrong terminology. Upon the completion of the 3 weeks, ~70% of student groups could successfully isolate the five components.During the experiment, it was observed that most students had acquired proper liquid-liquid separation techniques from previous organic chemistry laboratory courses. In the event that separations would not work, rotovap-evaporation procedures were implemented to “check” for substances and progress. Some groups even implemented solid-liquid separation techniques via filtration of precipitates.After the isolation process, students successfully characterized their components using traditional spectroscopy techniques such as IR and NMR. In addition to providing melting points for each substance. The substances were therefore identified via many reproducible methods through a straight comparison to literature published spectra and melting points. The low risk high learning potential of this experiment, makes it ideal for any class that involves upper level majors in spectroscopy.
4. Conclusions
- This experiment was successfully performed by students in structural chemistry course, an advance laboratory course for students who had already taken two semesters of organic chemistry. The students were delighted to know that no matter which extraction solvent they chose to start their separation protocol they achieved separation. We are currently developing a guided-inquiry experiment for separation of a six-component mixture.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML