-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2018; 6(5): 159-163
doi:10.5923/j.jlce.20180605.04

Geometric Isomerism in Octahedral Complexes: Synthesis, Characterization, and Reaction of Trans-Dichlorobis(ethylenediamine)cobalt(III) Chloride
Jocelyn Pineda Lanorio1, Jerry Gomez Lanorio2
1Department of Chemistry, Illinois College, Jacksonville, IL, USA
2Division of Mathematics, Science and Physical Education, Lincoln College, Lincoln, IL, USA
Correspondence to: Jocelyn Pineda Lanorio, Department of Chemistry, Illinois College, Jacksonville, IL, USA.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The synthesis of the coordination complex trans-dichlorobis(ethylenediamine)cobalt(III) chloride is a classic experiment and can provide an opportunity for inclusion of diverse concepts if additional activities are incorporated. This laboratory experiment outlines the use of modern instrumentation such as UV-Vis spectrophotometer for thermal ligand substitution monitoring, and the magnetic susceptibility balance for confirmation of the electron configuration of the cobalt(III) center in trans-dichlorobis(ethylenediamine)cobalt(III) chloride.
Keywords: Geometric isomerism, Octahedral cobalt(III) complexes, Aquation, Ligand substitution, Oxidation-reduction
Cite this paper: Jocelyn Pineda Lanorio, Jerry Gomez Lanorio, Geometric Isomerism in Octahedral Complexes: Synthesis, Characterization, and Reaction of Trans-Dichlorobis(ethylenediamine)cobalt(III) Chloride, Journal of Laboratory Chemical Education, Vol. 6 No. 5, 2018, pp. 159-163. doi: 10.5923/j.jlce.20180605.04.
Article Outline
1. Introduction
- Disubstituted planar and octahedral complexes exhibit both cis and trans stereochemistry. Figure 1 shows an example of octahedral geometric isomers. Octahedral cobalt(III) complexes that are low-spin d6 configuration are diamagnetic and considered stable, [1] having a filled t2g subshell. Due to the relative inertness of octahedral cobalt(III) metal centers, [1] their ligand substitution and isomerization reactions are slow compared to the complexes of many other transition metals. This allows us to monitor the substitution of chloride by water in the green trans-dichlorobis(ethylenediamine)cobalt(III) chloride, trans-[Co(en)2Cl2]Cl complex. The aquation product, [Co(en)2Cl(H2O)]Cl2, is purple in color and has cis geometry. [2]
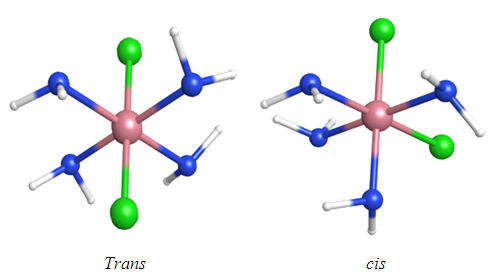 | Figure 1. Example of geometric isomers with octahedral geometry |
2. Experimental Section
- Customized side arm test tubes (Figure 2) were fabricated at Illinois College Department of Chemistry by the laboratory manager, Andy Stice. Alternatively, these can be purchased from several manufacturers such as Eisco labs, Haines Educational, and OnlineScienceMall, among others. Reagents were obtained from commercial sources and used as received. Thermal monitoring of the ligand substitution was carried out using Cary-60 UV-Vis spectrophotometer. The cuvette is immersed into hot water bath and transferred every 2 minutes to the UV-Vis spectrophotometer for a wavelength scan. Students were provided with an experimental handout and asked to answer the following prelab questions:1) Draw the structure of the complexes, trans-[Co(en)2Cl2]Cl and cis-[Co(en)2Cl(H2O)]Cl2.2) Balance the oxidation-reduction reaction involved in the synthesis of trans-[Co(en)2Cl2]Cl. Co2+ + H+ + H2O2 → Co3+ + H2OWhich species is the oxidizing agent? Which is the reducing agent?
 | Figure 2. Customized side arm test tubes |
2.1. Synthesis of Trans-Dichlorobis(ethylenediamine)co-balt(III) Chloride
- A modified procedure from Szafran, Pike, and Singh [4] for the synthesis of trans-dichlorobis(ethylenediamine) cobalt (III) chloride, trans-[Co(en)2Cl2]Cl, was performed. CoCl2.6H2O (300 mg, 1.26 mmol), water (2.5 mL), and 10% ethylenediamine (1 mL) were added to the side arm test tube. Dropwise addition of 0.300 mL of a 30% H2O2 solution was then done, followed with dropwise addition of 0.600 mL of concentrated HCl. A Pasteur pipet was inserted through a rubber stopper with a drill hole, to serve as air inlet, Figure 3. The test tube was then clamped in a hot water bath. Air was drawn slowly and continuously through the solution by connecting the side arm of the test tube to a vacuum source (aspirator or hood vacuum) by way of a water trap using appropriate tubing, Figure 4. The reaction was heated and oxidized until green crystals were apparent. Additional water should be added if the test tube is almost dry but has no formation of green crystals. The mixture was cooled in ice bath and then filtered by suction. The green crystals were washed twice with 2-mL portions of cold methanol, and then two 2-mL portions of cold diethyl ether. The filtrate that contains the HCl co-solvate (additional crystalline product) was discarded. The crystals were placed on a small watch glass and heated in an oven at 110°C until the next lab session.
 | Figure 3. A Pasteur pipet was inserted through a rubber stopper with a drill hole, to serve as air inlet |
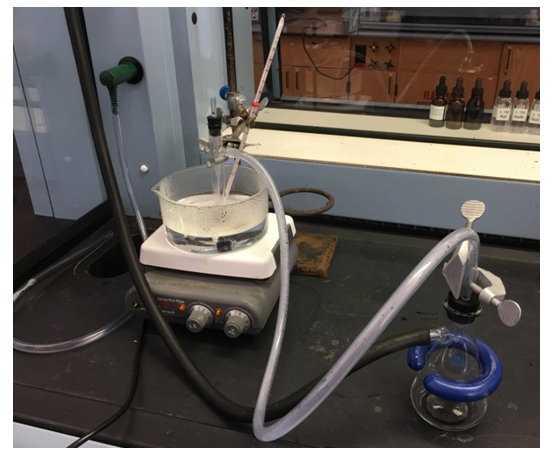 | Figure 4. Microscale synthesis of trans-[Co(en)2Cl2]Cl complex using a test tube with side arm attached to water-trap then vacuum source |
2.2. Characterization
- The green trans-[Co(en)2Cl2]Cl compound was weighed, and the percentage yield was obtained. The product was characterized by IR and UV-Vis spectroscopy. The IR spectrum of the product, Figure 5, was acquired using the Varian 640-IR FT-IR spectrophotometer. The molar extinction coefficient of trans-[Co(en)2Cl2]Cl was determined by preparing 5 mL aqueous solutions of 0.02, 0.04, 0.08, 0.12, 0.16 M trans-[Co(en)2Cl2]Cl. The UV-Vis spectra were obtained from 700 to 300 nm using the Cary-60 UV-Vis spectrophotometer.
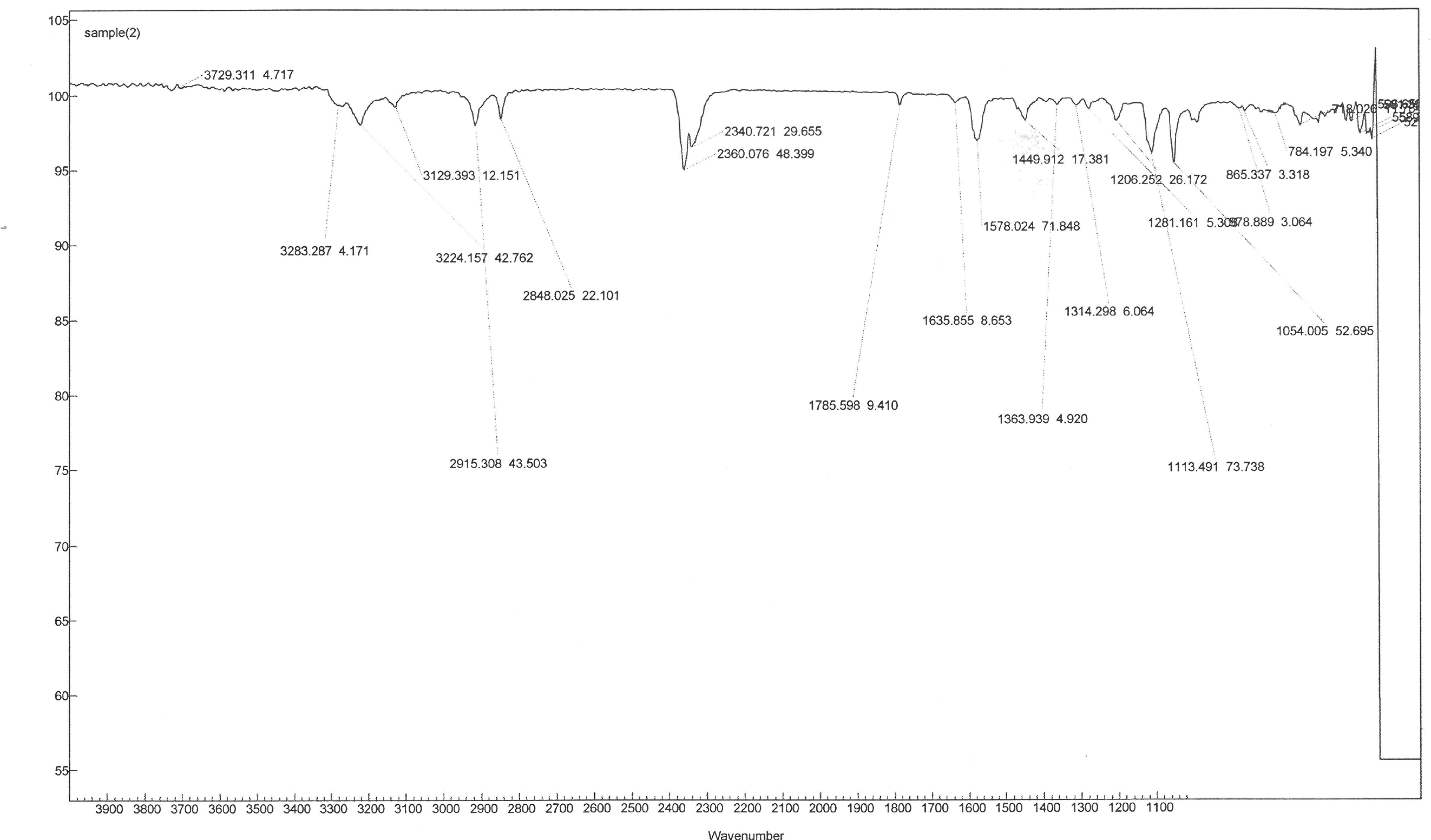 | Figure 5. IR spectrum of trans-[Co(en)2Cl2]Cl |
2.3. Aquation of trans-[Co(en)2Cl2]Cl: Synthesis of Cis-Dichlorobis(ethylenediamine)cobalt(III) Chloride
- UV-Vis spectrophotometer was used to observe the isomerization/aquation process at 80°C.A glass cuvette was filled with a few milliliters of 0.016 M aqueous trans-[Co(en)2Cl2]Cl solution. The cuvette was placed in a hot water bath then transferred to the UV-Vis sample holder for wavelength scan from 700 to 300 nm, in 2-minute intervals. Chloride substitution with water is complete once the solution turns purple permanently. The resulting solution contains the cis-[Co(en)2Cl(H2O)]Cl2 complex. The UV-Vis spectra display the absorption maxima for trans-[Co(en)2Cl2]Cl and cis-[Co(en)2Cl(H2O)]Cl2, Figure 6.
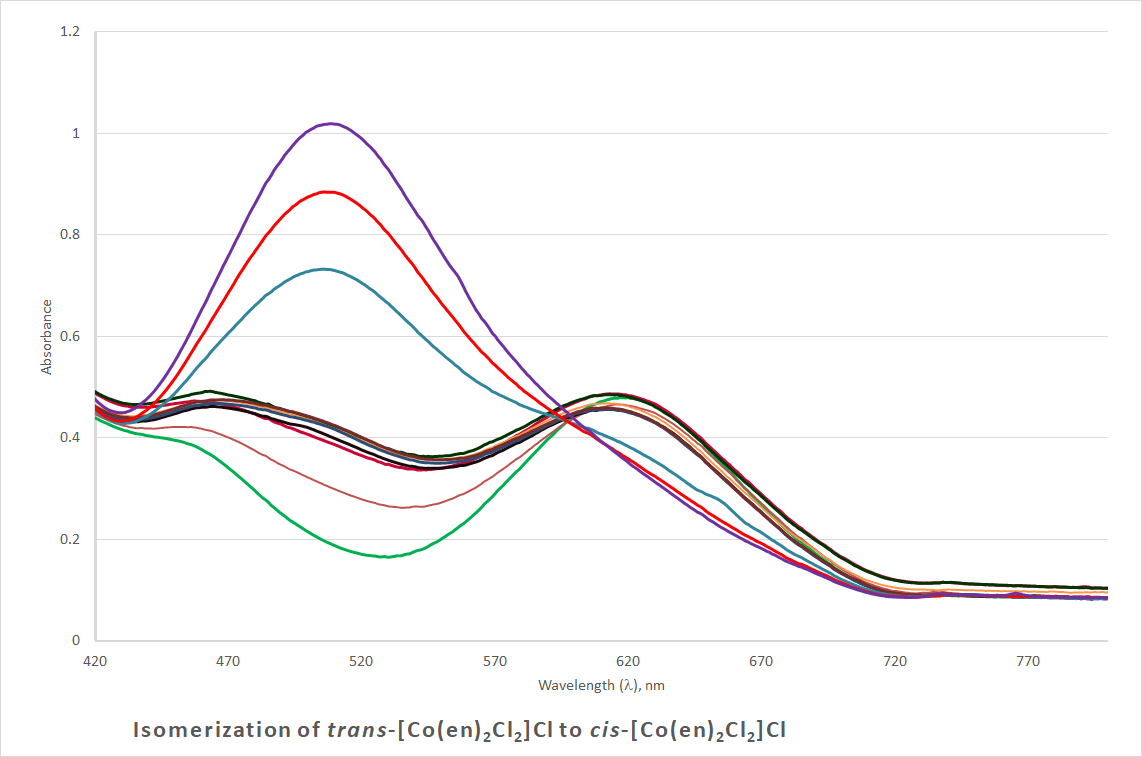 | Figure 6. UV-Vis spectra for the water substitution of chloride in trans-[Co(en)2Cl2]Cl |
3. Results and Discussion
- The set-up for the microscale synthesis of trans-[Co(en)2Cl2]Cl is shown in Figure 4. The initial and final colors of the mixture are shown in Figure 7. The final product trans-[Co(en)2Cl2]Cl obtained after filtration during the first lab meeting is shown in Figure 8. Typical student yields for the synthesis of trans-[Co(en)2Cl2]Cl were in the range of 45 to 50%.
 | Figure 7. Initial and final color of the mixture |
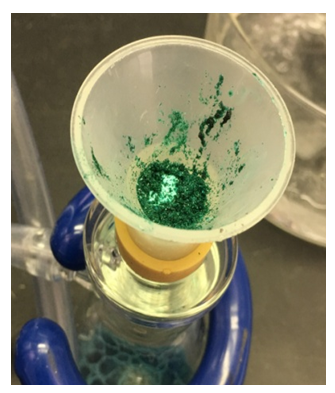 | Figure 8. Trans-[Co(en)2Cl2]Cl |
 | Figure 9. Cis-[Co(en)2Cl(H2O)]Cl2 complex |
 | Figure 10. Negative reading from magnetic susceptibility balance for the trans-[Co(en)2Cl2]Cl complex |
4. Conclusions
- This lab was successfully carried out by CH 332 Advanced Inorganic students in the Fall 2017 at Illinois College. The negative magnetic moment confirmed the successful oxidation of Co(II) to Co(III), and the d6 electron configuration of the cobalt(III) center. The IR spectrum showed the NH2 vibrational modes of the coordinated ethylenediamine ligand. The maximum absorption wavelengths obtained from UV-Vis spectral analysis of trans-[Co(en)2Cl2]Cl and cis-[Co(en)2Cl(H2O)]Cl2 are 618 nm and 509 nm, respectively. Students realized the importance of further use of modern instrumentation in monitoring reaction and determination of kinetic parameters, as noted in their lab reports.
ACKNOWLEDGEMENTS
- The experimental data presented in this work was obtained by Chan Myae Lin Latt and the CH 332 Advanced Inorganic Chemistry students, with the equipment and facilities of Illinois College Department of Chemistry.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML