-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2018; 6(5): 156-158
doi:10.5923/j.jlce.20180605.03

Esterification, Purification and Identification of Cinnamic Acid Esters
Anuradha Liyana Pathiranage1, Leah J. Martin2, Macy Osborne1, Kirsten Meaker1
1Department of Chemistry, Austin Peay State University, Clarksville, USA
2Department of Chemistry, Columbia State Community College, Columbia, USA
Correspondence to: Anuradha Liyana Pathiranage, Department of Chemistry, Austin Peay State University, Clarksville, USA.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Fisher esterification is a topic covered in most second semester undergraduate organic chemistry courses. This article outlines the synthesis of several esters produced via Fisher esterification of trans-cinnamic acid and various alcohols. The esters are purified using column chromatography, a separation technique typically introduced in the first semester. The products are characterized using Nuclear Magnetic Resonance (NMR) spectroscopy. Based on the identification of the esters produced, students determine the unknown starting alcohol and write the correct mechanism of the reaction. This laboratory project was successfully carried out with second semester organic chemistry laboratory students.
Keywords: Fisher esterification, Column chromatography, Nuclear Magnetic Resonance spectroscopy
Cite this paper: Anuradha Liyana Pathiranage, Leah J. Martin, Macy Osborne, Kirsten Meaker, Esterification, Purification and Identification of Cinnamic Acid Esters, Journal of Laboratory Chemical Education, Vol. 6 No. 5, 2018, pp. 156-158. doi: 10.5923/j.jlce.20180605.03.
1. Introduction
- Most organic chemistry laboratory manuals include esterification experiments [1-4]. Fischer esterification is an organic chemistry reaction that is taught in lecture and often carried out in the organic chemistry laboratory. Typically students start with a known acid and known alcohol and produce a pleasant-smelling ester, that is considered to be the unknown [5-8] In this undergraduate lab, students carry out a Fisher esterification synthesis with cinnamic acid and one of multiple simple alcohols, such as ethanol, propanol, butanol, iso-butanol, hexanol. All students work with the same acid, trans- cinnamic acid, but are given different alcohols (ROH) in the presence of sulphuric acid as the catalyst (equation 1). The students are not only identifying what the “unknown ester” produced, but they are also determining which alcohol was used to synthesise this ester based on knowledge of the mechanism, as well as NMR interpretation of the ester.
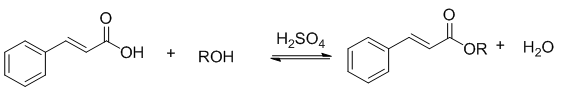 | Equation 1. Esterification reaction of cinnamic acid with unknown alcohols |
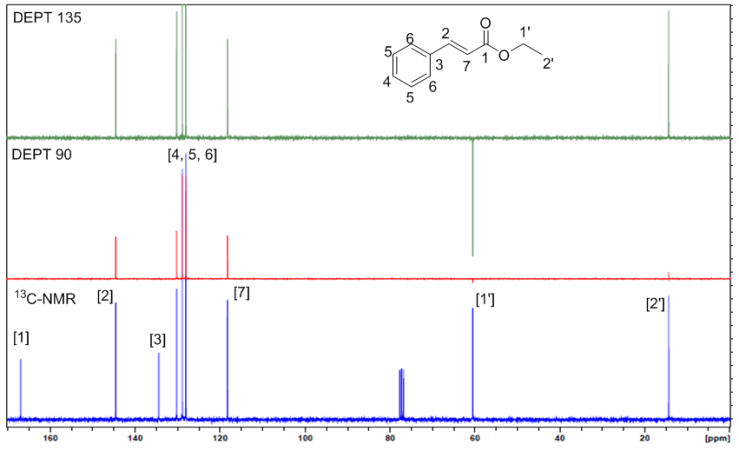 | Figure 1. Overlay of 13C-NMR, DEPT 90 and DEPT 135 of ethyl cinnamate from bottom to top |
2. Method
- Students typically work in pairs, but this experiment is suited for individuals as well. During the first laboratory session, students are assigned an unknown alcohol and all are given the same acid, trans-cinnamic acid as shown in Figure 2. The esterification reaction is carried out in the presence of sulfuric acid as describe in the procedure provided in the supplementary materials. The work-up removes most of the unreacted acids and alcohol, but further purification is used the following lab period.
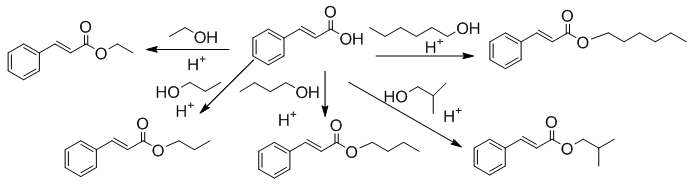 | Figure 2. Trans-cinnamic acid with different types of alcohols |
3. Conclusions
- This lab was successfully carried out in second semester undergraduate laboratory section with 22 students at Austin Peay State University. The students seemed to enjoy discovering the unknown esters using new spectroscopy techniques. This lab is more in-depth than the general verification lab. Not only do students identify the unknown ester produced, but they have to identify the starting alcohol as well. For future work, we would like to partner with area community colleges, where the students do not have access to the instrumentation, to see if student engagement increases and if there is a shift in attitude toward the lab in general.
ACKNOWLEDGEMENTS
- This work was supported by the Austin Peay State University Chemistry Department’s operating budget.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML