-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2018; 6(5): 148-155
doi:10.5923/j.jlce.20180605.02

Teaching Chemical Equilibria: A Contextualized Scientific Method and Forensic Chemistry Class
Murilo S. da S. Julião1, Silvia H. B. G. Rodrigues1, Lucia B. S. Andrade2, Luisa C. Melo3
1Laboratory of Applied Analytical Chemistry, State University of Valley of Acaraú (UVA/CE), Sobral-CE, Brazil
2Laboratory of Experimental Biology, State University of Valley of Acaraú (UVA/CE), Sobral-CE, Brazil
3CECITEC, State University of Ceará (UECE), Tauá-CE, Brazil
Correspondence to: Murilo S. da S. Julião, Laboratory of Applied Analytical Chemistry, State University of Valley of Acaraú (UVA/CE), Sobral-CE, Brazil.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This paper presents an experimental class involving forensic chemistry topics and principles of the scientific method in the investigation of a fictitious death case, where the students can understand the chemical reactions and respective equilibria involved in the case. Therefore, the aim of this experimental class is to provoke students to examine the interrelationships between the concepts of chemical equilibria, by observing the occurrence of reversible chemical reactions and the equilibria involved, in each experimental step. The practical activity was executed based on the discovery methodology. Experimental classes generally arouse the interest of undergraduates in chemistry, and this particular class increased the students' interest in forensic chemistry, and contextualized the chemical reactions and equilibria for crime-solving, which is a common theme in popular media such as books, films, and plays. From the reports of the students, it was affirmed that this experimental class, which was based on a rediscovery technique, promoted interest and learning of chemical equilibria to a satisfactory level.
Keywords: Experimental class, Chemistry teaching, Discovery methodology
Cite this paper: Murilo S. da S. Julião, Silvia H. B. G. Rodrigues, Lucia B. S. Andrade, Luisa C. Melo, Teaching Chemical Equilibria: A Contextualized Scientific Method and Forensic Chemistry Class, Journal of Laboratory Chemical Education, Vol. 6 No. 5, 2018, pp. 148-155. doi: 10.5923/j.jlce.20180605.02.
Article Outline
1. Introduction
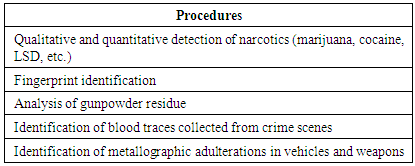
- Chemical equilibrium is an important concept in the understanding of various chemical interactions, but it is complex and considered one of the most difficult themes in chemistry teaching, both in high school and university, [1, 2] given the large number of studies investigating the conceptual difficulties of students regarding chemical equilibria. Most studies identify the students' main misunderstandings: misunderstanding the differences between a complete and incomplete chemical reaction; [3-6] believing that the reverse reaction begins only when the direct reaction ends; [3-6] and difficulty in understanding the dynamic nature of equilibrium. [7, 8]The contextualization of chemistry teaching has emerged as a proposal in national curricula for improving secondary education, aimed at channeling the scientific information in a social context. Thus, chemistry should provide young learners with a broad vision of life, allowing them to reflect on current problems that exist in the world. Importantly, they should be capable of making a connection between chemistry and the subjects directly related to this science, and the issues that are routinely encountered in daily life. [9]Considering the importance of understanding the concepts of chemical equilibria and its ubiquity, this paper reports the application of an experimental class involving forensic chemistry, where principles of the scientific method were used to investigate a fictitious case. The goal is that the students understand chemical reactions and respective equilibrium involved in the different steps of the investigation.Forensic science is a multidisciplinary subject that involves biology, physics, mathematics, medicine, and chemistry, to assist in investigations related to legal issues. This field uses chemical techniques and concepts to investigate facts related to certain crimes. Forensic chemistry deals with field forensic investigations involving specialized chemistry to meet judicial requirements. This field also incorporates the disciplines of criminology and forensic medicine. [10]Table 1 lists examples of chemical analyses commonly used in forensic chemistry.
|
2. Experimental
2.1. Materials
- In this experiment, the following materials were used by each group of students: test tubes, droppers (1.0 mL capacity), spatulas, test tube holders, cotton swabs, acetylsalicylic acid (ASA) tablets, NaCl crystals, and solutions 0.10 mol.L-1 of Pb(NO3)2, HCl, K2CrO4, KSCN, and FeCl3. In addition, 1.0 mL of 1.0 mol.L-1 HCl was added to prevent hydrolysis of Fe3+. All reagents were purchased from Sigma-Aldrich®.
2.2. Sample Preparation for the Experimental Class
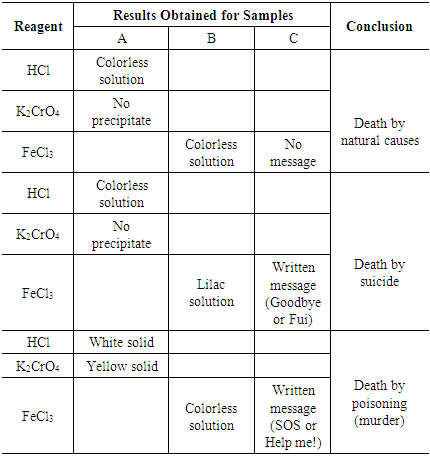
- A total of 10 samples were prepared, which simulated three different situations, i.e., samples whose analysis results could lead to the correct conclusion of the “causa mortis”:- Sample A (simulated urine): pineapple juice was filed in distilled water to 100.0 mL (1:100 (v/v) dilution), as it has the same color and appearance as urine. For the test result to be positive, 1.0 mL of 0.10 mol.L-1 Pb(NO2)3 was added to 1.0 mL of the simulated urine sample. In contrast, to provide a negative test result, 0.50 g of NaCl was added to 1.0 mL of sample A.- Sample B (white powder): for the positive sample, a small amount of pre-crushed aspirin tablets was added to a test tube. For the negative sample, 0.50 g of NaCl was added to the test tube.- Sample C (crumpled paper): the tip of a cotton swab, slightly moistened in KSCN solution, was used to write words on the surface of irregularly cut filter paper pieces, like "FUI! (goodbye in Portuguese) and "GOODBYE!" (indicating suicide) or "SOS” and “HELP ME!" (calls for help). For samples indicative of death by natural causes, no words were written on the paper.Note: To reveal the alleged message contained on paper, each group of students was instructed to gently rub the tip of another cotton swab, moistened with FeCl3 solution, on the paper.
2.3. Methodology
- Understanding the basic principles of the scientific method is not the learning goal of this study, but it is necessary for the preliminary approach to the experimental class (pre-laboratory). The basic concepts of the scientific method were presented to allow the students to execute and understand the practical activity proposal involving chemical equilibria.The pre-lab started with the following question for the students: "What is the scientific method?". Subsequently, the method was explained by saying, doing, and teaching emphasizing certain principles and methodology to reach an end. The scientific method can be said to be the path taken by the scientist when he seeks scientific "truths". [12]The scientific method is widely used in the physical and life sciences and consists of studying a phenomenon in the most rational way possible to avoid mistakes, always seeking evidence and proof for the hypotheses, conclusions, and affirmations. It is therefore a “set of approaches, techniques, and processes to formulate and solve problems in the objective acquisition of knowledge”.During the pre-lab, steps involved in the scientific method were presented to the students:a) Observation: a phenomenon is observed, and curiosity often develops based on the observation.b) Experimentation: the same phenomenon is triggered several times while monitoring all possible variations and values. At this stage, careful measurements are taken.c) Establishment of scientific laws: after analyzing the experimentation data, a scientific law can be formulated, which is a generalization that relates to the studied phenomenon. It is important to note that the scientific law is not an explanation of why, but only a description (preferably mathematical) of the phenomenon.d) Elaboration of hypotheses: at this stage, explanations are generated for the phenomenon and its related laws. The explanation (why?) often leads to the creation of a "model". The simplest hypothesis or model should be chosen as the most probable explanation for the phenomenon studied. A model is a formal description of a phenomenon, which can make predictions. In this context, a hypothesis is an assumption that is made in an attempt to explain the problem.e) Establishment of a thesis: if the hypothesis is proved by experimental testing, it becomes a thesis. A thesis is a proven hypothesis. From the theses, models are created.f) Elaboration of a theory: a theory is a set of theses that explain the same phenomenon or some related phenomena and that has been tested and proven by a large number of experiments.The importance of establishing suitable methodology for experimental classes was highlighted by Hodson, [13] indicating that if there are no research situations proposed in experimental classes, there will be no difference in the type of classes planned by the teacher for expository, demonstrative, or practical experiments. Therefore, difficulty in understanding the chemistry course content, such as chemical equilibria, may be more directly related to the methodology used in chemistry teaching, than to the type of lecture, whether experimental or theoretical.The methodology used for the execution of practical classes was based on the “discovery methodology”, since it favors scientific knowledge construction through directed activities that stimulate doing and thinking. In other words, they involve the students in the manipulation of materials, stimulating them to reflect, make decisions, and draw conclusions. [14] The experimental teaching of chemistry using discovery methodology leads the students to develop their skills and scientific attitudes. The discovery methodology, using the scientific method formally or informally, allows the students to constantly developed their observation, measurement, comparison, hypothesis formulation, graphing, data analysis, and interpretation skills to draw conclusions.Rediscovery is a technique that commonly appears in this methodology. It is a didactic tool by which the teacher proposes practical activities and, through experiments, leads students to observe and interpret results, enabling them to draw their own conclusions. [15] In this technique, students work without knowledge of the final objectives, and only discover the objectives when a certain stage is reached, or when the experiment is finished.In this experimental class, the teacher provided the materials, assisted the students in assembling the experimental setup, and encouraged them to make observations and draw their own conclusions, but the actual experiments were performed by the students. Experiments based on aspects of forensic chemistry were performed in a group of thirty undergraduate students in the experimental analytical chemistry discipline at the State University of Valley of Acaraú, in Sobral-CE, Brazil.The class was divided into 10 groups of 3 students and the groups were divided into three sections: initially they were given a script whose introductory text covered the principles of the scientific method and forensic chemistry, followed by the materials and methods. An expository class (pre-laboratory) was given so that the class could perform all steps of experiments with minimal errors, because according to Mayer, [14] sometimes the development of the activity requires prior knowledge, as some students may lack the contextual information.The steps of the class align with the rediscovery technique, that is, it is up to the teacher to identify an objective subject that justifies the realization of the experimental class; verify its feasibility and adequacy, and provide the materials and bibliography to be used in class proposal. [14]In this proposal, each student should act as a forensic chemist to elucidate the “causa mortis” of a person. In this fictitious scenario, the forensic chemist collaborated with the local police department, which made a summary report of the death that was being investigated:(i) a male approximately 50 years old was found dead in his apartment;(ii) a declaration from a friend of the deceased stated that for more than three days, the victim had not returned telephone calls, and so he decided to go to the apartment. He found the door locked, sensed a strong smell coming from inside the apartment, and immediately called the police. The informant also stated that the dead friend, complying with medical advice, always had aspirin® (ASA) tablets on hand to relieve heart pain (angina);(iii) according to the police, who arrived after 30 min, they had to break into the apartment and found the man lying on the ground;(iv) forensic experts were called in and attested that the man had been dead for at least 72 h.As determined by the forensic experts, the possible causes of death were either: (i) poisoning by the ingestion of some heavy metal salt (arsenic, lead, or mercury); (ii) suicide caused by ingestion of a large excess of drugs (e.g., anticonvulsants, antidepressants, or antihypertensive drugs); or (iii) death due to natural causes (myocardial infarction, stroke, etc.).To accept or disprove these hypotheses, the teacher (representing the forensic expert), handed out, for each group of students (forensic chemists), samples (evidence) found close to the victim, to be analyzed in the laboratory.Regarding the hypothesis of poisoning by unconscious ingestion of some heavy metal salt, the presiding doctor collected a sample of urine (sample A) from the bladder of the deceased and sent it to the forensic chemists.The forensic chemists should be made aware that two pieces of evidence (clues) were found next to the victim and may be of fundamental importance in the elucidation of the cause of death: a) a glass of water next to a plastic bottle containing powder (sample B) on the table in the apartment, and b) a piece of blank crumpled paper (sample C) in the dead man's hand.Thus, students acting as forensic chemists performed the following procedures:- Physical description of sample A (color, turbidity, etc.). In a test tube, 1.0 mL (approximately 20 drops) of sample A and 1.0 mL of HCl were added and the mixture was observed. If this test provided a positive result, color change, precipitate formation, or gas release, students mixed sample A with 1.0 mL K2CrO4. On the other hand, if the initial test performed with HCl was negative, students continued to the direct powder analysis (sample B);- Physical description of sample B (color, solubility, etc.). In another test tube, a small amount of sample B and 1.0 mL of distilled water were added, stirred, and mixed together. The resulting mixture was observed and described. Subsequently, 5 drops of FeCl3 were added to the mixture, with a lilac color indicating the presence of acetylsalicylic acid (ASA). If the solution remained colorless or was colored differently, this would indicate the absence of ASA;- For sample C, the students, in addition to describing its physical characteristics, were prompted by the teacher to question whether there was any meaning of the piece of paper, since a more experienced forensic expert would immediately suspect that the victim would have written a message. Therefore, this suspicion should be considered by the forensic chemists, who proceeded as follows: the tips of cotton swabs were dampened with FeCl3, passed gently over the paper, and the occurrence of any reaction was observed and noted.Table 2 contains the guidelines provided to the students, with the steps of each test of the samples and possible results.
|
3. Results and Discussion
- At the end of the experimental class, possible phenomena related to the observations were explained to the students. For the groups of students who tested sample A and observed changes in the initial stage (positive test), the explanation given by the teachers was that the appearance of a white solid (precipitate) when mixing sample A with HCl was due to precipitation of PbCl2. After the addition of K2CrO4, formation of a yellow precipitate (PbCrO4), occurred, and can be used to identify the presence of Pb2+ in the systematic analysis of cation groups. Ionic reactions presented to the students were as follow:
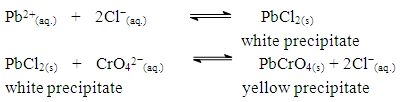 The solubility product, Ksp, of PbCl2 and PbCrO4 are 1.7 x 10-5 and 3.0 x 10-13, respectively, [16] and the students were shown that the Ksp values can be used as a reference to predict precipitate formation in a mixture of solutions. In this test, the final concentrations of chloride and chromate ions were high, so both precipitates were formed, since product of the ion concentrations exceeds the Ksp of each compound. This resulted in a supersaturated solution and precipitation occurred to reestablish equilibrium conditions.Thus, it was possible to demonstrate to the students a practical example of precipitation equilibria of Pb2+ as a function of Ksp values of PbCl2 and PbCrO4. Students were able to understand that the rate at which the insoluble compounds are formed is related to the concentrations of the ionic species and their Ksp values.The dynamics of the experimental class favors the reinforcement of previous concepts related to reversible chemical reactions studied by the students in the general chemistry course. In addition, some discrepancies encountered in laboratory activities, such as those discussed by DeMeo, [17] may contribute to understanding these concepts. In this case, the qualitative investigation of two precipitation reactions (i) the formation of lead chloride (PbCl2) from lead nitrate and hydrochloric acid solutions, and (ii) the formation of lead chromate (PbCrO4) from the PbCl2 precipitate and potassium chromate solution — were used to introduce the concept of chemical equilibrium.These reactions were selected because they are easy to implement, fit well with the students' prior knowledge and favor educational improvements. Procedural and motivational questions inherent in the experimental class are likely to attract students interested in an environment conducive to the process of constructing ideas. [18]Although the definitions of reversible and irreversible reactions and equilibrium constants were previously addressed, the concept of a "dynamic model" was used to explain these subjects in a more attractive manner, by presenting the principal macroscopic characteristics of chemical reactions at equilibrium.For the groups students who tested sample B (reaction with 0.10 mol.L-1 FeCl3) and observed a lilac-colored solution, it was explained that the coloration confirmed the presence of ASA, according to the ionization of ASA in aqueous medium and subsequent reaction with Fe3+ ions (Schemes 1 and 2) explained during class.
The solubility product, Ksp, of PbCl2 and PbCrO4 are 1.7 x 10-5 and 3.0 x 10-13, respectively, [16] and the students were shown that the Ksp values can be used as a reference to predict precipitate formation in a mixture of solutions. In this test, the final concentrations of chloride and chromate ions were high, so both precipitates were formed, since product of the ion concentrations exceeds the Ksp of each compound. This resulted in a supersaturated solution and precipitation occurred to reestablish equilibrium conditions.Thus, it was possible to demonstrate to the students a practical example of precipitation equilibria of Pb2+ as a function of Ksp values of PbCl2 and PbCrO4. Students were able to understand that the rate at which the insoluble compounds are formed is related to the concentrations of the ionic species and their Ksp values.The dynamics of the experimental class favors the reinforcement of previous concepts related to reversible chemical reactions studied by the students in the general chemistry course. In addition, some discrepancies encountered in laboratory activities, such as those discussed by DeMeo, [17] may contribute to understanding these concepts. In this case, the qualitative investigation of two precipitation reactions (i) the formation of lead chloride (PbCl2) from lead nitrate and hydrochloric acid solutions, and (ii) the formation of lead chromate (PbCrO4) from the PbCl2 precipitate and potassium chromate solution — were used to introduce the concept of chemical equilibrium.These reactions were selected because they are easy to implement, fit well with the students' prior knowledge and favor educational improvements. Procedural and motivational questions inherent in the experimental class are likely to attract students interested in an environment conducive to the process of constructing ideas. [18]Although the definitions of reversible and irreversible reactions and equilibrium constants were previously addressed, the concept of a "dynamic model" was used to explain these subjects in a more attractive manner, by presenting the principal macroscopic characteristics of chemical reactions at equilibrium.For the groups students who tested sample B (reaction with 0.10 mol.L-1 FeCl3) and observed a lilac-colored solution, it was explained that the coloration confirmed the presence of ASA, according to the ionization of ASA in aqueous medium and subsequent reaction with Fe3+ ions (Schemes 1 and 2) explained during class.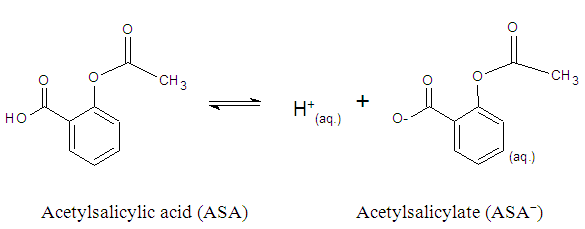 | Scheme 1. Ionization equilibrium of ASA. [16] |
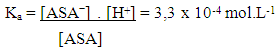 | (1) |
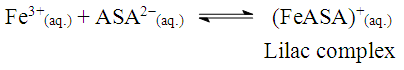 | Scheme 2. Formation of the iron (III) acetylsalicylate complex |
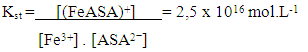 | (2) |
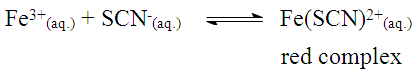 | Scheme 3. Formation of the iron (III) thiocyanate complex |
 | (3) |
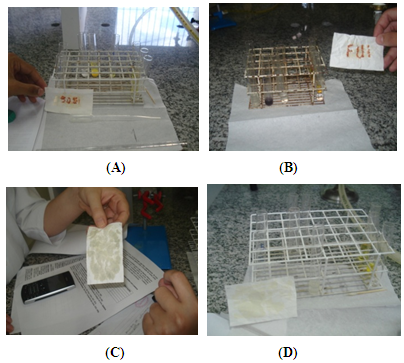 | Figure 1. Photos of the paper containing messages revealed with FeCl3: "SOS" (A), "FUI” (goodbye, B), without messages (C) and (D) |
|
|
4. Conclusions
- Experimental classes generally arouse interest of undergraduate chemistry students, but this class especially increased students' interest and curiosity regarding forensic chemistry and was able to contextualize different kinds of chemical equilibria with current themes that unfortunately are not pleasant (unresolved crimes). These themes are part of daily life and are often the subject of books, films, and theatrical performances.This experimental class included more intense participation and discussions compared to those of previous practical classes. This was also observed during the pre-lab class, and success was achieved in the orderly execution of experiments. This, is a crucial factor to achieve the correct conclusion to unravel the mysterious death in the fictitious scenario. All students accepted the teacher's instructions to observe the steps of the scientific method, seek explanations, and confirmation hypotheses.With regard to the theoretical approach of the scientific method, a lack of maturity was observed because of the novelty of this concept. However, rediscovery improved the learning outcomes with regard to chemical equilibria to a satisfactory level, since the students were initially provoked to execute and observe assays involving chemical equilibria and to draw conclusions regarding importance of chemical equilibria in the assays performed by forensic experts.
ACKNOWLEDGEMENTS
- Authors thank the agencies: CNPq and CAPES and for financial support and for research fellowship in chemistry teaching (PIBID), respectively.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML