-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2018; 6(4): 133-139
doi:10.5923/j.jlce.20180604.09

An Accelerated Chemical Weathering Assay: Sulfation of Acinipo Limestone in a Humid and SO2 Rich Environment
Aimee R. Taylor1, 2, 3, Juan A. Ocaña-González2, Juan L. Pérez-Bernal2
1Department of Chemistry, University of York, York, YO10 5DD, United Kingdom
2Departmento de Química Analítica, Facultad de Química, Universidad de Sevilla, c/ Prof. García González s/n, Seville, Spain
3Center for Communicable Disease Dynamics, Harvard T.H. Chan School of Public Health, Boston, MA, USA
Correspondence to: Juan L. Pérez-Bernal, Departmento de Química Analítica, Facultad de Química, Universidad de Sevilla, c/ Prof. García González s/n, Seville, Spain.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Sulfation is one of the main factors of calcareous stone deterioration. In a bid to better preserve our cultural and historic heritage for future generations, the sulfation of limestone from the archeological site of the ancient Roman city of Acinipo was investigated. To simulate sulfation, limestone samples from Acinipo weresubjected to accelerated weathering by chemical attack of SO2 in a humid environment. Weathering provoked the substitution of the calcite matrix for crystals of both calcium sulfate dihydrate and calcium sulfite hemihydrate. The results offer interesting leads into further investigation, while demonstrating the educational utility of the proposed methodology.
Keywords: Limestone, Accelerated Chemical Weathering, Sulfation, Monuments and Architecture Damage
Cite this paper: Aimee R. Taylor, Juan A. Ocaña-González, Juan L. Pérez-Bernal, An Accelerated Chemical Weathering Assay: Sulfation of Acinipo Limestone in a Humid and SO2 Rich Environment, Journal of Laboratory Chemical Education, Vol. 6 No. 4, 2018, pp. 133-139. doi: 10.5923/j.jlce.20180604.09.
Article Outline
1. Introduction
- Throughout history stone has been used in architecture and art on account of its longevity. Unfortunately, the changes in morphology and composition which result from the interaction of stone with its environment are often aesthetically and functionally detrimental to monuments and buildings. In unpolluted environments, reactions are often extremely slow, unfolding over centuries. In the twentieth century however, atmospheric pollution, due to increased industrial activity, has had a dramatic effect on the rate of anthropogenic alteration, resulting in an alarming increase in the deterioration of our artistic and cultural heritage [1]. In order to better inform the actions required to prevent further damage and to safeguard our heritage for future generations, it is very important to elucidate the mechanisms of stone degradation.Stone degradation results from an interplay of numerous alteration factors, both natural and anthropogenic. It is also dependent upon the inherent lithology of the stone, the function it is serving and the treatments to which it has been subjected. The indicators of alteration are equally diverse and dependent upon the weathering factors. The overall mechanism is thus a potential combination of mechanical, physical, chemical and biological processes, and as such extremely complex to determine [2].One of the principal causes of damage to monuments and architecture in Europe and North America is sulfation of carbonaceous stone, such as limestone [3, 4]. Sulfation occurs primarily in industrialized and urban areas, where atmospheric sulfur dioxide abounds. Oxidation of atmospheric sulfur dioxide results in sulfur trioxide which dissolves in liquid water or vapor giving rise to sulfurous or sulfuric acid which in turn attacks the stone and provokes the substitution of the calcite matrix for calcium sulfate dihydrate, commonly known as gypsum [1, 3]. Gypsum is relatively stable but moderately soluble in water and thus liable to dissolution by rain water and consequent wash out. In areas not exposed to mechanical stress, the formation of gypsum can result in the buildup of crusts, which vary in depth from microns to millimeters. Such crusts often appear black when impregnated with highly porous oil-fired carbonaceous particles, which catalyze sulfation and nucleate the genesis of gypsum crystal [5]. Moreover, the adhesion between the healthy stone and the gypsum layer is poor, such that the crusts are prone to separation from the stone’s surface, thereby exposing fresh stone [1]. To better understand sulfation, studies of different types of limestone in sulfur dioxide rich atmospheres have been conducted [6-11]. These studies are commonly known as accelerated weathering assays. Accelerated weathering assays facilitate mechanistic elucidation by allowing the control of variables in isolation [6]. However, in doing so they also obliterate the possibility of synergism. Since no single factor ever acts in isolation in a real environment, weathering assays can never fully simulate the mechanism of true weathering. Moreover, the magnitude of the weathering factors is many times greater than in real environments [3]. Accelerated weathering tests are also an important tool for the evaluation of the behavior of conservation and restoration treatments [12, 13]. Consequently, although accelerated weathering assays are very important tools, they are also oversimplified approximations, and results must be considered comparatively with caution and skepticism. Acinipo (Ronda, Andalucía, Spain) is an important archeological site dating back to 202 BC. It boasts impressive ruins of a roman theatre and baths overlooking the Spanish Sierra de Grazalema [14]. The stone under current investigation is found in abundance over excavations on the site and is the same as that found in the Formation of Setenil [14, 15]. A full petrographic description of Acinipo limestone is included in the report of the findings by IAPH [15]. It is a fine grained, sandy textured, biochemical limestone. It is macroscopically homogeneous (with the exception of a few shells and grains of quartz) facilitating the preparation of small stone samples. It is pale beige; most likely on account of the small iron content in the form of limonite.The main objective of the present work is to propose a methodology to study the effect of a sulfur dioxide rich environment on samples of limestone from the ancient roman city Acinipo. To investigate the overall process of sulfation, previously characterized stone samples from Acinipo were exposed to a humid environment with high sulfur dioxide concentration. Changes in the stone’s composition were followed over time using different analytical techniques.
2. Materials and Methods
2.1. Chemicals and Samples
- Stock samples of Acinipo limestone were prepared as 60 mm3 blocks and left to equilibrate in laboratory conditions (20°C, 55-60% RH).Twice deionized water and analytical grade reagents were used throughout.Barium acetate precipitating solution was prepared by dissolving 20g of barium acetate in 75 cm3 10 N acetic acid and 25 cm3 5% aqueous arabic gum solution, which was subsequently filtered.Iodine solution was prepared from 2g potassium iodide and ca. 0.15g potassium iodate (accurately weighed to 0.1 mg) in acid media (10 ml of 0.5 mol dm-3 H2SO4 dissolved in ca. 50 ml water).Sulfurous acid solution was prepared by diluting 500 cm3 of sulfurous acid containing 5% SO2 in mass in 150 cm3 water.
2.2. Chemical Characterization
- The following methods were based on those proposed by A. Martín [16].Loss by CalcinationWith the aid of a muffle oven and an analytical balance, the loss of CO2 by calcination was determined. Specifically, 0.7 g of finely ground stone, previously dried at 110°C, was accurately weighed into a porcelain crucible of known mass and calcined at 950°C for two hours before cooling in a desiccator. The process was repeated until the obtained mass was constant and the result expressed as the percentage mass lost.Acid attack: determination of insoluble silica and preparation of solution for inductively coupled plasma atomic emission spectroscopy (ICP)Soluble stone solutions were prepared in triplicate for ICP analysis by acid attack and the percentage mass of the insoluble quartz (SiO2) determined by calcination.One gram of finely ground stone, previously dried at 110°C, was accurately weighed into a 500 cm3 beaker equipped with a watch glass and stirrer. 35 ml HCl (39%, d = 1.19 g cm-3) and 1 ml HNO3 (d = 1.39 g cm-3) were then added to the beaker with care. Any solid fragments were broken up with the stirrer and the solution heated gently in the covered beaker to dryness on a sand bath. The process of adding acid media and heating to dryness was repeated three times; HNO3 was omitted from the third addition. Water was added to the stone residue and the solution filtered through ash free analytical filter paper (pore size 140 μm) into a 250 ml volumetric flask, ensuring full transference. The paper was subsequently transferred to a crucible of known mass (pre-calcined at 950°C for two hours) and dried in an oven at 110°C. It was then calcined with a Bunsen burner, taking great care to avoid loss of ash, and subjected to 1000°C in the muffle oven for an hour, before being left to cool in a desiccator and weighed. The elemental composition of the solution was determined by atomic emission spectrometry by Plasma ICP (Horiba Jobin Yvon, model Ultima 2) courtesy of CITIUS, Universidad de Sevilla.
2.3. Accelerated Weathering Assay
- The procedure followed was based on the 2002 European standard for natural stone tests: Determination of resistance to ageing by SO2 action in the presence of humidity [17].Several 20 mm × 10 mm × 5 mm stone samples, each supported on a watch glass, were placed on a ceramic support above 650 cm3 sulfurous acid solution in a 6 dm3 desiccator (Figure 1).
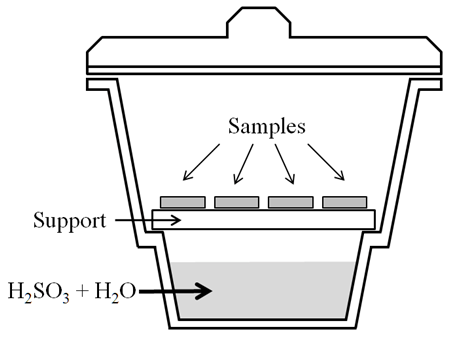 | Figure 1. Schematic of the accelerated weathering assay setup |
2.4. Water Assay
- The water content of stone samples in both natural and pre-saturated states was determined as a function of time in a desiccator containing 650 ml water. In order to mimic the experimental conditions of the weathering chamber, water content was determined at atmospheric temperature and pressure. Water content (W) was calculated from the percentage mass difference between dry stone (Ms) and stone in its state of interest (M):W = 100 x (M – Ms) / Ms.Dry stone was dried at 60°C, since this was the temperature used in the evaluation of the weathering assay (section 2.5).
2.5. Analytical Methods: Weathering Evaluation
- As above, the following methods were based on those proposed by A. Martín [16].Mass Difference After predetermined exposure times, weathered samples were dried in an oven at 60°C to ensure the evaporation of moisture without loss of crystallization water. Once dry, the stone samples were left to equilibrate to their natural state in laboratory conditions and their mass measured on an analytical balance (MF). The percentage mass difference (% MD) of each sample was then calculated with respect to its pre-exposure mass (M0), % MD = 100 x (MF – M0) / M0.The mass difference is dependent upon the conversion of CaCO3 to CaSO3.½H2O and CaSO4.2H2O, both of which have a higher molecular weight than CaCO3.ConductivityHaving calculated the mass difference, ca 5 g of stone was ground in an agate mortar and sieved to a particle size smaller than 200 µm. Approximately 1 g of ground stone was accurately weighed into an airtight plastic container, to which 100 ml of water was accurately pipetted. The solution was then stirred for 24 hours before leaving to the solid fraction to settle overnight.The corrected conductivity (K), which corresponds to 1g of stone in 100 ml of solution, was determined by a GLP 32 Crison Conductimeter as follows: K = (a – b) / m,where a (µS cm-1) is the conductivity of the aqueous stone solution, b (µS cm-1) is the conductivity of a blank solution prepared as per the test solution but without stone, and m (g) is the mass of the stone in the aqueous solution.Turbidimetric Sulfate DeterminationTo a 50 cm3 volumetric flask containing 10 ml aqueous stone solution diluted in 30 ml of water, 1 cm3 of barium acetate precipitating solution was added and the solution made up to 50 cm3 with water.The flask was agitated for 2 minutes and left to rest for a further 10 minutes before measuring the absorbance at 425 nm using a UNICAM UV 500 UV/visible spectrometer. A blank was prepared in exactly the same manner but omitting the aqueous stone solution. The results were calculated by comparison with a calibration curve, constructed from a standard solution of SO42-. Sulfite DeterminationThe sulfite content of the stone was determined by iodometric back titration. Iodate reacts with iodide to yield iodine:IO3- + 6H+ + 5I- → 3I2 + 3H2O.A known excess of iodine solution (in I3- form) was added to a known weight of the solid stone and was left in the dark for 15 minutes for quantitative sulfite to sulfate oxidation:SO32- + I3- + H2O → SO42- + 3I- + 2H+The remaining un-reacted iodine was then back-titrated using previously standardized 0.1 M sodium thiosulfate solution:2S2O32- + I3- → S4O62- + 3I-
3. Results
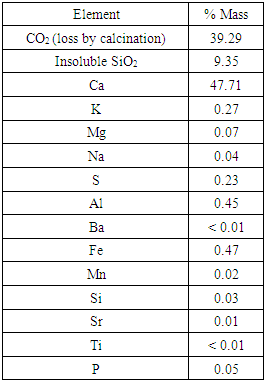
3.1. Chemical Characterization of Acinipo Limestone
- The results of chemical characterization (Table 1) were in agreement with those from IAPH [15]. That is, the limestone proved to be almost pure calcite with a relatively high quartz content (9.35%).
|
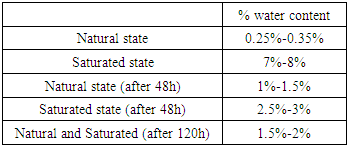
3.2. Water Assay
- The water content of Acinipo stone samples in their natural state in laboratory conditions was 0.25% to 0.35% by weight. The water content of saturated samples ranged from 7% to 8%. During exposure to the humid environment, saturated samples lost water while the water content of unsaturated samples increased (Table 2). After 120 hours, the water content of all samples ranged from 1.5% to 2%.
|
3.3. Weathering Assay
- During the accelerated weathering assay the samples showed progressive de-cohesion, as well as the appearance of macroscopic fissures, which were more evident in the pre-saturated samples. A pre-saturated sample exposed for 626 hours suffered a complete rupture resulting in fragmentation.Figure 2 shows the increase in mass of the samples over time. There was little qualitative difference between trends across pre-saturated samples and those exposed in their natural state. Regardless of state, there was an initial phase of rapid mass increase during the first 120 hours, reaching an average 10% mass increase. Followed by a slower increase, reaching 14% after 624 hours.
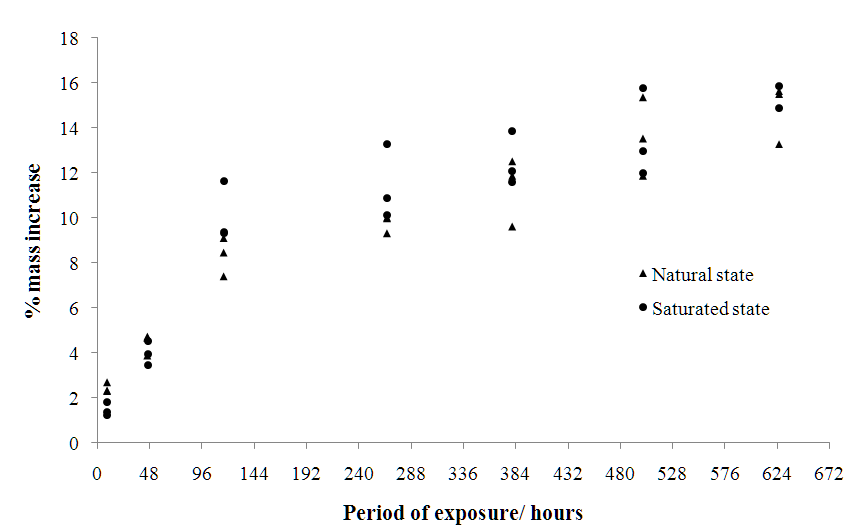 | Figure 2. Percentage mass increase of weathered stone samples over time |
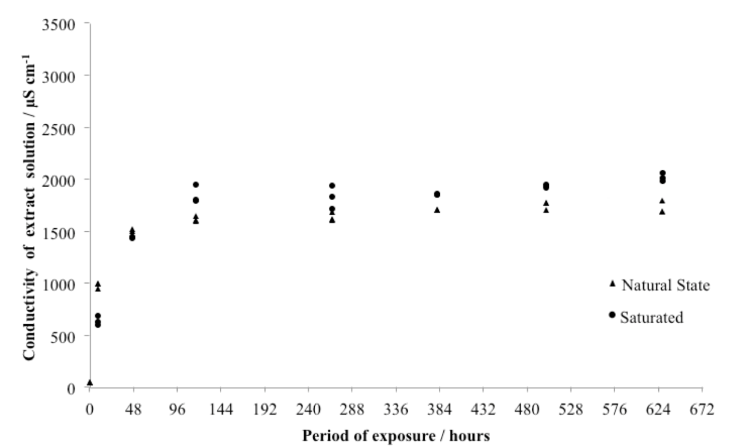 | Figure 3. Corrected conductivity of 100 ml aqueous solution containing 1 g of over time |
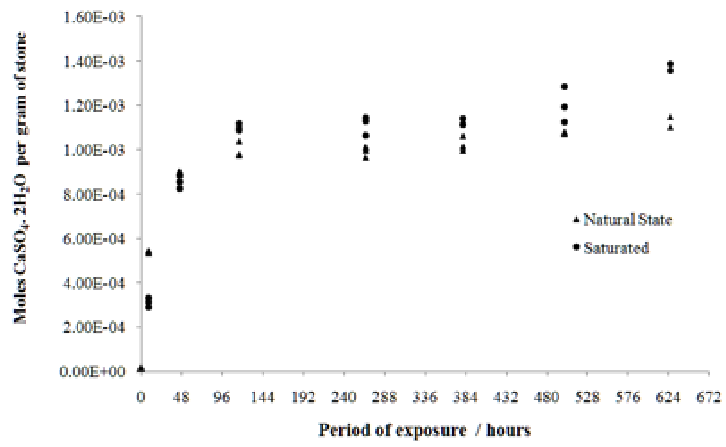 | Figure 4. Number of moles of calcium sulfate in 100 ml aqueous solution containing 1 g of stone, as a function of time |
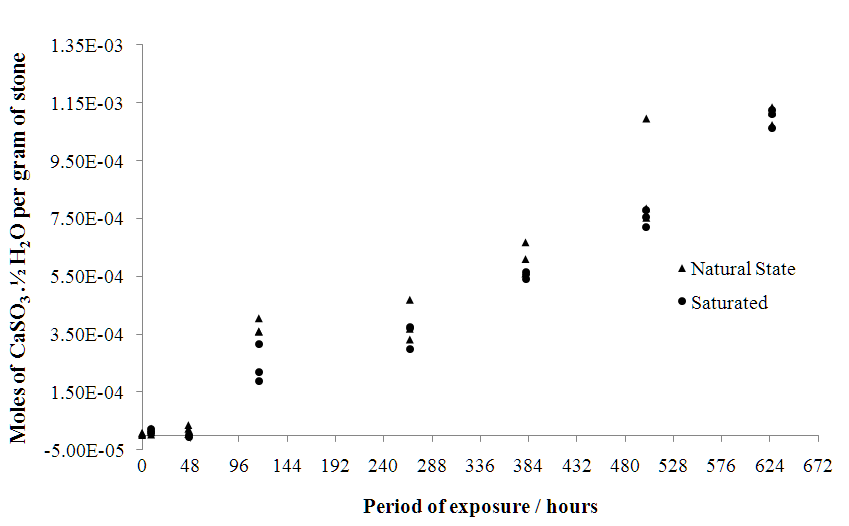 | Figure 5. Number of moles of calcium sulfite in a 100 ml aqueous solution containing 1 g of stone, as a function of time |
4. Discussion
- The sulfation of limestone from Acinipo in a humid and SO2 rich environment was investigated. The results support the existence of an interaction between the limestone and the weathering environment. Figures 3, 4 and 5 provide evidence that the reaction involved the decomposition of the calcite matrix to gypsum and calcium sulfite, thereby testifying that both species are thermodynamically more stable than CaCO3 in the conditions found within the weathering chamber. Over the exposure times studied, the formation of calcium sulfite over time was approximately linear, whereas the rate of formation of gypsum declined. This can be explained assuming a rapid conversion of calcium sulfite to calcium sulfate by S(IV) to S(VI) oxidation.Conductivity of aqueous stone solution increases with the concentration of the electrolytes (ions of soluble salts). The soluble salts commonly found as a result of sulfation are: gypsum (CaSO4.2H2O), MgSO4, and Na2SO4 in varying degrees of hydration and calcium sulfite in the form of CaSO3·1/2H2O [3]. Since Acinipo limestone is almost pure calcite (Table 1) the observed increase in conductivity (Figure 3), was likely due primarily to the formation of gypsum. The formation of calcium sulfite appeared to have little bearing on the increase in conductivity, concurrent with its low solubility in water. On the contrary, the mass increase (Table 2) is compatible with the conversion of CaCO3 to both calcium sulfate and sulfite. Weathering not only produces an increase in atomic mass, but also affects the hygroscopicity of the stone, which itself is a function of the chemical and morphological alteration induced by the attack.
5. Conclusions
- In this paper we present results from the sulfation of limestone in a humid environment. The proposed methodology allowed characterization of the involved processes. The different chemical processes involved (acid-base and redox reactions, gas-solid and gas-liquid reactions, and quantitative aspects) also illustrate the connection between chemical principles and the preservation and study of cultural heritage. With a simple and un-expensive experimental set-up that was easy to implement in an educational laboratory, and with the use of classical (redox titrations) and instrumental (turbidimetry, conductimetry) techniques, this methodology is accessible, informative and interesting for undergraduate students.
ACKNOWLEDGEMENTS
- The authors wish to acknowledge to the Microanalysis service of CITIUS, “Centro de Investigación, Tecnología e Innovación” of the University of Seville for the ICP facilities.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML