-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2018; 6(4): 128-132
doi:10.5923/j.jlce.20180604.08

Homemade Reflectometer for Brightness Measurements of Metal Coatings: Application in Laboratory Electroplating Experiments
José Luis Olloqui-Sariego, Emilio Roldán
Department of Physical Chemistry, University of Sevilla, Profesor García González, Sevilla, Spain
Correspondence to: Emilio Roldán, Department of Physical Chemistry, University of Sevilla, Profesor García González, Sevilla, Spain.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The brightness measurement of coated surfaces provides valuable information of the quality of coatings. This paper describes the development of a simple, inexpensive and portable homemade reflectometer apparatus for applying in brightness measurement of a variety of coatings. The reflectometer comprises three main parts: the electronic system, a sample holder and a voltmeter. The electronic system includes the illumination unit and light sensor unit. The illumination unit contains the light source consisting in four optional LED of different emission wavelengths in series with current limiting resistors and a potentiometer that allow us to regulated the intensity of the incident light. The light sensor unit consists in a TSL 250 light-to-voltage optical sensor. The sample holder is designed to include two angles of lighting and two angles of measuring reflected radiance, 30° and 60°, that allows brightness measurements of coatings over the range of medium to high gloss. The reliability of the present instrument was assessed in short preparatory nickel electrodeposition experiments by studying the influence of organic brightener concentration on the brightness of nickel coatings. It can be observed that the reflectometer apparatus developed allows to distinguish the relative brightness in samples with very short brightness differentiation, thus evidenced a very good sensitivity. These experiments can be used to introduce both optical instrumentation and electrochemistry concepts related with the surface treatment in industrial processes for chemistry and engineering students.
Keywords: Reflectometer, Brightness, Metal Coating, Electroplating, Brighteners, Optical Instrumentation
Cite this paper: José Luis Olloqui-Sariego, Emilio Roldán, Homemade Reflectometer for Brightness Measurements of Metal Coatings: Application in Laboratory Electroplating Experiments, Journal of Laboratory Chemical Education, Vol. 6 No. 4, 2018, pp. 128-132. doi: 10.5923/j.jlce.20180604.08.
Article Outline
1. Introduction
- A coating is a covering that is applied to the surface of an object, usually referred to as the substrate. Coating is largely used in a variety of industries for decorative and/or functional purposes. One of the most used coating techniques is the plating in which a metal is deposited on a surface. In particular, electroplating, the deposition of metal coatings onto conducting surfaces by making these in the cathode in an electrolytic cell, with a suitable electrolyte containing heavy metal ions [1], is used extensively in the industry for decorative, engineering, and electroforming purposes. This electrochemical process is of immense interest for chemistry and engineering students. However, teaching laboratory electrodeposition demonstrations are scarce [2, 3]. The quality of metal deposits in the industry is tested by microstructure assessment and some thermal and mechanical properties such as corrosion resistance, hardness, ductility and stresses. The characterization of abovementioned properties requires the use of specific and complex instrumentation that not are available in teaching laboratory.The efficiency of electroplating experiment for undergraduate students is usually estimated from the relationship between current, time (i.e. the electric charge used) and the mass gained from the electroplated metal by using the Faraday´s laws of electrolysis. Complementary to this methodology, gloss measurement appears as a good parameter for qualitative assessment of the quality of coatings. Gloss is the attribute of surfaces that causes them to have shiny or lustrous, metallic or matte appearances. This parameter can be measure by non-destructive optical methods based on light reflection by shining a known amount of light at a surface and quantifying the reflectance. Bright deposits are characterized by the fact that, by impacting on its surface a luminous beam, light is strongly reflected on a single direction. Thus, the brightness of a metal coating increases with increasing the reflection of incident light. In the same way, brightness measurement provides valuable qualitative information of the structure of the electrodeposit considering the well-known relationship between the roughness of deposits and brightness [4, 8].On the basis of the foregoing, in the present work we designed and constructed a simple, low cost and portable reflectometer apparatus for brightness measurement of coatings for using in teaching laboratory. To assess the reliability of this instrument, we have study the influence of brightener concentration on the gloss of electrodeposited nickel coatings. Moreover, the homemade apparatus has been successfully introduced in a class of approach to surface treatment by electrochemical method for undergraduate students.
2. Apparatus and Experimental
2.1. Reflectometer Setup
- Figure 1 shows the reflectometer electronic framework from different viewpoints. It can be observed that apparatus can be easily transportable because it small size. In the front view, it can be seen the LED selector, a light intensity controller and the optical fiber (OF) output(s) and input signals. The connections to the voltmeter and the power supply unit (PSU) are found in the back side of the reflectometer.
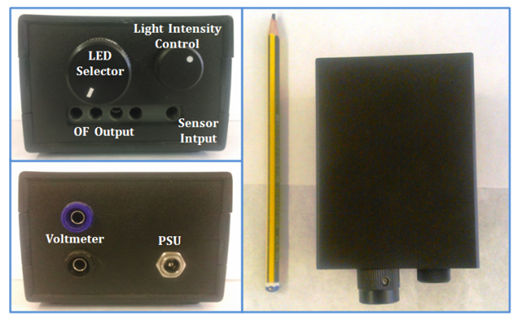 | Figure 1. Photos of the homemade reflectometer from three viewpoints |
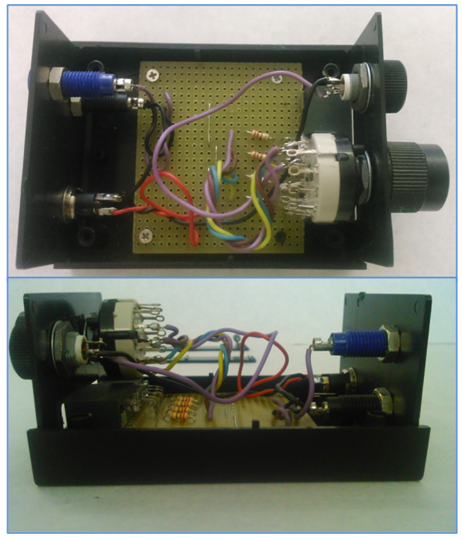 | Figure 2. Photos of the assembly of the electronic subsystem from two viewpoints |
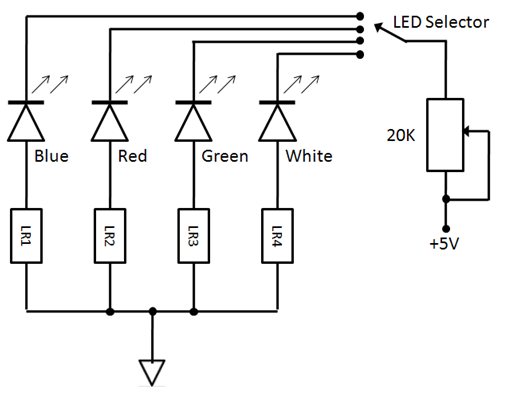 | Figure 3. Circuit Schematic of the illumination unit |
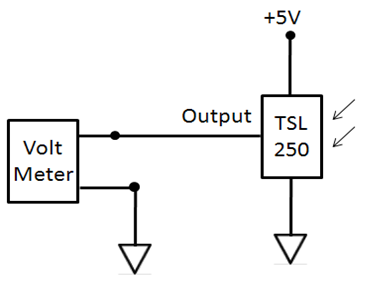 | Figure 4. Circuit Schematic of the light sensor unit |
2.2. Nickel Electroplating Experiments
- The experimental setup used to carry out the electroplating demonstrations includes a glass beaker containing the electrodeposition bath and a magnetic stirrer, hot plate stirrer and a variable AC/DC power supply. The nickel electroplating solution (200mL) was a typical modern Watts bath [9] containing nickel sulphate (200 g/L, NiSO4•6 H2O), nickel chloride (45 g/L, Ni2Cl•6 H2O) and boric acid (40g/L, H3BO3). The electrolytic solutions were prepared with Millipore water and all the chemicals were analytical reagent grade (from Panreac). Each ingredient of the electrolyte has a definite function [10]. Nickel sulphate is used to provide the major part of the nickel ion content because it is the least expensive salt of nickel with a stable anion which is not oxidized at the anode, reduced at the cathode, or volatilized. The chloride is introduced to improve anode behavior by reducing polarization, but high chloride contents also permit higher finer-grained and uniformity deposits on the cathode. In the absence of a buffer, nickel deposits are prone to be hard, cracked, and pitted. Boric acid is the preferred buffering agent used and provides an acid medium (pH = 3.4 ± 0.2), which increases the overpotential of hydrogen evolution and the equilibrium potential will be displaced sufficiently to enable nickel deposition to occur.To assess the influence of brightener on the brightness of the nickel electrodeposits, non-toxic additives o-sulfobenzoic imide (saccharin), paratoluene sulfonamide and benzene sulfonamide (all of them 98%, from Abcr) were used. The electrodes were printed circuit board (PCB) plates sized 20 x 25 mm as cathode and a nickel rod anode. The use of this sacrificial anodes ensures that the composition of the solution ideally remain unchanged. Constant current density electrolysis were carried out during 10 minutes at 60 mA cm-2 and at 45 ± 3°C. Prior to plating, the cathode was cleaned with nitric acid 2M solution to remove any oxide films and grease. The plating solution was continuously stirred during the course of the experiments, providing uniform deposits. After that, the coated PCB plates were thoroughly washed with water and ethanol and dried.
2.3. Brightness Measurements
- The reflectometer setup for brightness measurements comprises three parts (see figure 5), the electronic system (illuminator and sensor), a sample holder and a voltmeter. The light is transmitted between the reflectometer and the sample holder by using 1,5 mm PMMA jacketed OF, from Edmund Scientific. These OFs are connected to the reflectometer using bored nylon M8 screws. Nylon screws are faced to the LEDs and sensor through a machined PVC piece. The voltmeter is connected to the reflectometer with common copper cable.
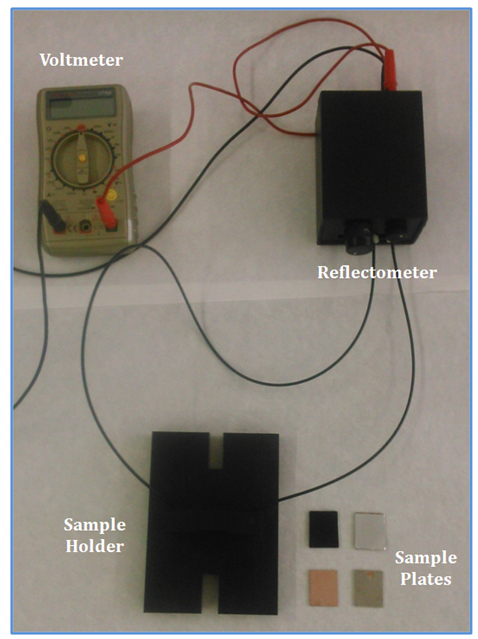 | Figure 5. Photos of the device for brightness measurements |
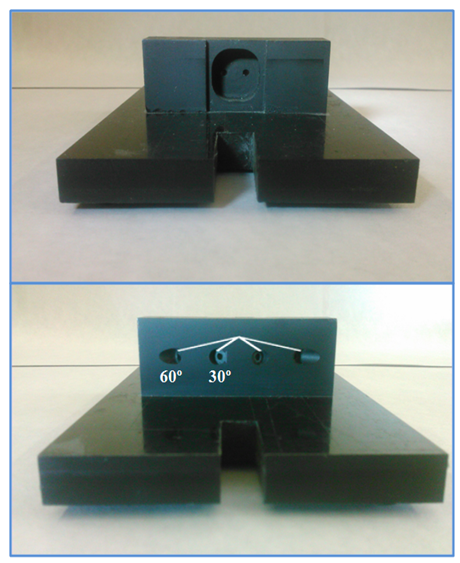 | Figure 6. Photos of the sample holder from front and back views |
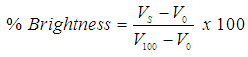 | (1) |
3. Results and Discussion
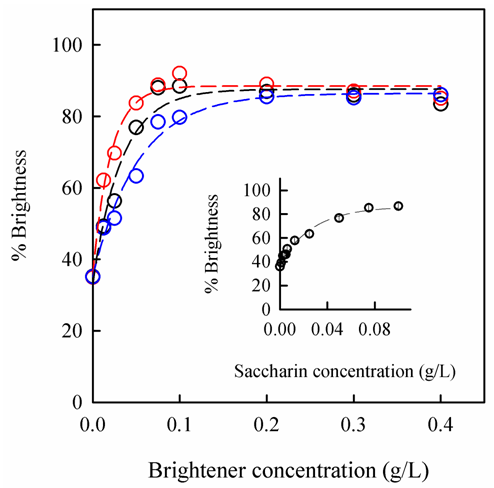
- In order to test the reliability of the system we assessed the influence of the brightener additives on the brightness of nickel electrodeposited coating. It has been probed that the use of organic additives in metal electrodeposition and, in particular in nickel electroplating, improves the levelling and brightening of the metal electrodeposited coatings [11-15]. Short preparatory nickel electrodeposition experiments were developed varying the brightener concentration. The variation of the relative brightness of nickel coatings as a function of additives concentration is depicted in figure 7. In the absence of brightener, the relative brightness is low, likely due to the deposition of a very rough deposit that causes a dispersion of the reflected light in all directions. This result reflects the well-known relationship between the roughness of deposits and brightness. Interestingly, a very small addition of brightener to the electroplating solution significantly increases the relative brightness of nickel deposits. The maximum reflectance is achieved at additive concentration of 0.1 g/L. At higher concentrations, the brightness of the nickel deposits remains unchanged or even slightly decreases when benzene sulphonamide is used. It is well known that the presence of additive in electroplating bath improves the roughness of the metal coating because of the diminution of the grain size [16, 17]. Bearing on mind that, among others, the roughness of the electrodeposited metal is directly correlated to its brightness, we can conclude that the smoothest and uniform surface is achieved at saturation level of the relative brightness.
4. Conclusions
- A simple, low cost and portable reflectometer apparatus for brightness measurement of coatings has been designed and constructed for using in teaching laboratory for chemistry and engineering students. The reflectometer comprises three main parts: the electronic system, a sample holder and a voltmeter. This apparatus allows non-destructive and rapid evaluation of the quality of coatings over the range of medium to high gloss, particularly suitable for metallic coatings. The reliability of the present instrument was assessed in short preparatory nickel electrodeposition experiments by studying the influence of organic brightener concentration on the brightness of nickel coatings, showing a very good sensitivity. These experiments allow the students to evaluate simply, rapidly and visually, the effect of the operating electroplating conditions on the quality of metal coatings. Additionally, these experiments can be used to introduce both optical instrumentation and electrochemistry concepts related with the surface treatment in industrial processes.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML