Domingo González-Arjona
Physical Chemistry Department, University of Sevilla. (Spain)
Correspondence to: Domingo González-Arjona, Physical Chemistry Department, University of Sevilla. (Spain).
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Cite this paper: Domingo González-Arjona, Chemistry Laboratory in Graduate Projects, Journal of Laboratory Chemical Education, Vol. 6 No. 4, 2018, pp. 0-0. doi: 10.5923/j.jlce.20180604.00.
Article Outline
PrefaceThe Chemistry Graduation Project (Bachelor’s Thesis), is an important part of the Chemistry undergraduate curriculum [1]. It provides students with an opportunity to integrate their accumulated knowledge and hone their experimental skills acquired throughout the programme. Application of their knowledge of Chemistry through interdisciplinary projects performed in a group should surely be encouraged and implemented more extensively as the setting in which such projects are carried out is likely to be close to what they will encounter in their future professional careers.However, various difficulties may arise in conducting interdisciplinary undergraduate projects, especially where it involves coordination among the different areas of specialization within Chemistry itself (Analytical, Inorganic, Organic, Physical Chemistry,…) and time management as students have to organize their activities to fit within a rather tight schedule. Another potential challenge relates to budgetary constraints, always a limiting factor in investigations of an experimental nature. Especially in a subject like Chemistry, experimentation is a critical step in the advancement of knowledge within the general framework of the Scientific Method, see figure 1.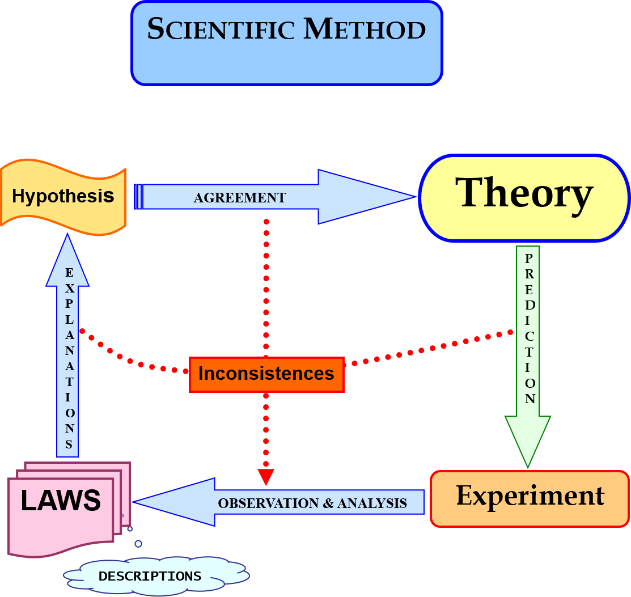 | Figure 1. Experimentation in the framework of Scientific Method |
Nevertheless, the benefits to the student’s development easily outweigh the challenges of planning, organizing and developing the projects.A number of research groups have contributed to this Special Issue of the Journal of Laboratory Chemical Education, 2018. In total, nine papers have been compiled covering various aspects of the Chemistry laboratory experience which can be used as a basis for designing undergraduate projects.These papers exhibit all aspects of proper scientific methodology: revision of available literature on the specific topic, replication of published data, hypothesis testing, verification of experimental procedures with literature, acquisition of experimental data, and even development of some basic instrumentation tailored to meet the specific demands of the project.The paper by Mosier-Boss [2], highlights the importance of replication in science by analyzing the track formation in solid state nuclear detectors. The project described here extended over several years and was carried out by different groups at different levels. The subject area is not entirely free from controversy, but rather than this being a reason to shun the topic, it can be seized upon to offer an opportunity to gain insight into the scientific method by seeing it in action. The experiments illustrate the importance of replication of results reported in the literature as a means of quality control within the framework of scientific methodology, especially in the case of unexpected or anomalous results.Two papers from Martin et al. [3], deal with the commonly employed technique of potentiometric titration. In the first paper, the authors compare the advantages and disadvantages of Gran II and Schwartz methods for the determination of the titration equivalent point and pKa. The other paper deals with the associated potentiometric errors in the case of titration curves for weak acids/bases. They derive a general expression which can be applied to all possible combinations in acid/base strength. The method is applied to the determination of the acetic acid content of commercially available vinegar.Three other papers from the same group [4], treat the difficulties in the spectrophotometric pKa determination when the system under study is a diprotic acid with two similar pKa values. The authors analyze, by using literature data, the sources of errors and propose different strategies (bilogarithmic and Poiser method) for minimizing these errors. The authors suggest a method for the evaluation of acidity constants that is checked with simulated data, literature data and using fresh data obtained in their laboratory.A review of possible undergraduate projects based on the Flow Injection Analysis technique is presented by Maraver et al. [5]. The automatization of this procedure is relatively cheap and can be easily carried out by using open-science methodology and recycled parts. This article offers useful suggestions for the design of interdisciplinary projects by combining aspects of chemistry, electronics, computer science, etc.In the same spirit, guided by the necessity to design and build equipment when operating under low-budget conditions, Olloqui-Sariego and Roldán [6] report on a simple, inexpensive and portable homemade reflectometer. The construction of the device, setup and commissioning is described in full detail. The reliability of the device is checked by measuring the gloss of metallic coatings generated by electrolysis under different experimental conditions. Owing to its interdisciplinary and multi-purpose nature, this smart device can also be used to develop a wide range of comprehensive graduation projects involving spectroscopy, electrochemistry, material science, etc.Taylor et al. [7], present an article in which the chemistry of the degradation of archaeological artefacts can be used as a theme around which to develop interdisciplinary projects. A methodology to study the effect of a sulfur dioxide-rich atmosphere on Roman artefacts is proposed. Also in this case, a simple and relatively cheap experimental set-up is employed.As this special issue once again demonstrates, the Journal of Laboratory Chemical Education, despite it having been in existence for a relatively short time, has provided a forum for publishing articles that suggest interesting lab experiments that enhance Chemistry student’s experience and that can be used as a basis for the design and development of more advanced undergraduate projects [8].
References
| [1] | López-Pérez, G.; Domínguez Pérez, M. M; González-Arjona, D.; González González, A. G.; Martín Valero, M. J; Fernández Torres, R.; Villar Navarro, M. and Vázquez Cabello, J. (2011). “The Development of New Approaches in the Bachelor Thesis Work in Chemistry”. @tic. revista d’innovació educativa. (nº 8). https://doi.org/10.7203/attic.8.1008. Accessed May 2018. |
| [2] | Mosier-Boss, P. A. and Forsley, L. P., “Energetic Particle Emission in Pd/D Co-deposition: An Undergraduate Research Project to Replicate a New Scientific Phenomenon” (2018), Special Issue JLCE. |
| [3] | Martín, J.; Barrios Ruiz, D. and Asuero, A. G., “Determination of the End Point in Potentiometric Titrations: Gran and Schwartz Methods”, and Martín, J.; Ortega Estévez, L. and Asuero, A. G., “On the Titration of a Weak Acid with a Weak Base: Application to the Potentiometric Determination of the Degree of Acidity of Vinegar”, (2018), Special Issue JLCE. |
| [4] | Martín, J.; Abengózar Suárez, I. and Asuero, A. G., “On the Existence of Two Species in Solution, Simple or Simultaneous Equilibria?”; and Martín, J.; Hidalgo Soria, A. and Asuero, A. G., “Spectrophotometric Evaluation of Acidity Constants: Can a Diprotic Acid Be Treated as a Monoprotic One?” and “Evaluation of Acidity Constants of p-Aminobenzoic Acid by Means of a Bilogarithmic Complete Method”, (2018), Special Issue JLCE. |
| [5] | Maraver, J. J.; Carbajo, J.; López-López, M. and Mozo, J. D., “The Flow Injection Analysis as an Opportunity to Develop Comprehensive Graduation Projects”, (2018), Special Issue JLCE. |
| [6] | Olloqui-Sariego, J. L.; Roldán, E., “Homemade Reflectometer for Brightness Measurements of Metal Coatings: Application in Laboratory Electroplating Experiments”, (2018), Special Issue JLCE. |
| [7] | Taylor, A.R.; Ocaña-González, J.A. and Pérez-Bernal, J.L., “An Accelerated Chemical Weathering Assay: Sulfation of Acinipo Limestone in a Humid and SO2 Rich Environment”, (2018), Special Issue JLCE. |
| [8] | Journal of Laboratory Chemical Education. Most downloaded articles. http://www.sapub.org/Journal/mostdownload.aspx?journalid=1139. Accessed May 2018. |



 Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML