-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2018; 6(3): 65-68
doi:10.5923/j.jlce.20180603.03

Novel Controlled Synthesis of CeO2 Hollow Microspheres by a Simple Hydrothermal Method: Undergraduate Experiments for Nanomaterials
Zhiyan Guo, Zhaobo Wang, Baoxiang Wang, Rupeng Zhao, Xinmeng Ning, Fangling Du
College of Materials Science & Engineering, Qingdao University of Science & Technology, Qingdao, China
Correspondence to: Zhiyan Guo, College of Materials Science & Engineering, Qingdao University of Science & Technology, Qingdao, China.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Wetting chemical method is a common method for the preparation of nano-materials, which are useful for experimental study by undergraduate students. CeO2 hollow microspheres have been successfully synthesized by hydrothermal decomposition reaction of Ce(NO3)3 on the surfaces of silica microspheres. The shell thickness of CeO2 hollow microspheres can be controlled from 20 nm to 50 nm by adjusting the concentration of Ce(NO3)3 in the reaction solution. The morphologies of CeO2 hollow microspheres were characterized by X-ray powder diffraction (XRD), transmission electron microscopy (TEM), field-emission scanning electron microscopy (FE-SEM).
Keywords: CeO2 hollow microspheres, Hydrothermal decomposition reaction, Morphologies
Cite this paper: Zhiyan Guo, Zhaobo Wang, Baoxiang Wang, Rupeng Zhao, Xinmeng Ning, Fangling Du, Novel Controlled Synthesis of CeO2 Hollow Microspheres by a Simple Hydrothermal Method: Undergraduate Experiments for Nanomaterials, Journal of Laboratory Chemical Education, Vol. 6 No. 3, 2018, pp. 65-68. doi: 10.5923/j.jlce.20180603.03.
Article Outline
1. Introduction
- Cerium oxide is a major compound in the rare earth family that has attracted considerable attention because of their vast technological applications, such as photocatalysts, [1-3] electrochemical cells, [4] Optical and Magnetic Properties, [5] gates for metal-oxide semiconductor devices and phosphors, [6-7] biomaterials, [8] sorbents for H2S removal, [9] and ultrviolet absorbents for sunscreens, [10] which are of great interest due to their wide applications, in particular, as redox or oxygen storage promoters in the three-way catalysts, catalysts for H2 prodution from fuels, and solid state conductors for fuel cells. [11-12] Cerium (IV) oxide, also known as ceric oxide, ceric dioxide, ceria, cerium oxide or cerium dioxide, is an oxide of the rare-earth metal cerium. It is a pale yellow-white powder with the chemical formula CeO2, which is insoluble in water. It is an important commercial product and an intermediate in the purification of the element from the ores. The distinctive property of this material is its reversible conversion to a nonstoichiometric oxide. Cerium Oxide is fluorite phase structure (Fig. 1), whose molecular weight is 172.115 g/mol and chemical formula is CeO2. The valence of cerium is +4, so it possesses strong oxidability. Its melting point and boiling point is 2400°C and 3500°C, respectively. CeO2 can be obtained by the oxidation of Ce2O3:2 Ce2O3 +O2 → 4 CeO2.Normally, CeO2 can not be reacted with the common acid. Furthermore, CeO2 occur the oxidation-reduction reaction with CO in the presence of high temperature:4 CeO2 + 2 CO → 2 Ce2O3 + 2 CO2 ↑The work presented here aims at the development of an integrated research-education activity on novel controlled synthesis of CeO2 hollow microspheres.
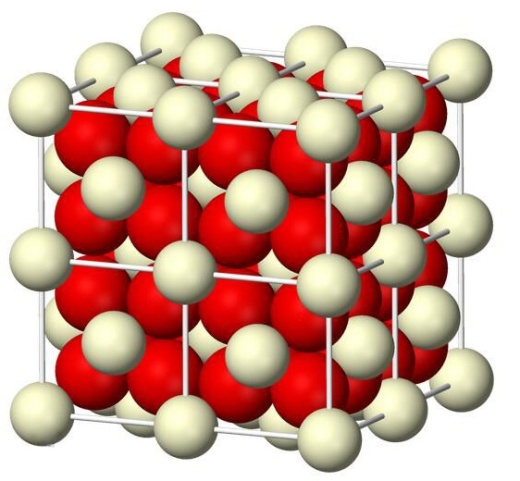 | Figure 1. The structure of cerium dioxide |
2. Experiment Description
2.1. Experiment Materials
- Cerous nitrate (Bide Chemical Reagent Limited Company, Shanghai, China), sodium hydrate (NaOH) (Hengda Fine Chemical Products Limited Company, Shanghai, China), anhydrous methanol (Tianjin Fuyu Fine Chemical Co. Ltd., Tianjin, China), aqueous NH4OH solution (Hengda Fine Chemical Products Limited Company, Shanghai, China) and tetraethylorthosilicate (TEOS) (Tianjin Fuyu Fine Chemical Co. Ltd., Tianjin, China) were purchased. All reagents were of analytical grade and used as received without further treatment. Distilled water was used in the experiment.
2.2. Instruments and Characterization
- The morphologies and sizes of the resulting CeO2 products were determined by field-emission scanning electron microscopy (FE-SEM, JSM 6700F), transmission electron microscopy (TEM, JEM-2000EX), The crystal structures of the resulting products were characterized by X-ray powder diffraction (XRD, D/MAX-500 X-ray powder diffractometer with Cu Kα radiation, λ=1.5418 Å at 40 kV and 70 mA) and an UV-vis spectrophotometer (Cary 500), respectively.
3. Experimental Procedure
3.1. Preparation of Silica Spheres
- The uniform silica spheres were synthesized using Stöber method. [13] 245 mL of concentrated aqueous NH4OH solution was added into the aqueous EtOH solution (EtOH 225 mL, H2O 10 mL) and the resulting solution was stirred vigorously for 30 min. Then 21 mL of tetraethylorthosilicate (TEOS) was added in the solution and was stirred for 4 hrs. The mixture was centrifuged, decanted and dried in 100°C.
3.2. Preparation of CeO2 Hollow Microspheres
- In a typical synthesis of CeO2 hollow microspheres, 0.868 g Ce(NO3)3·6H2O was dissolved in 60 mL deionized water containing 0.1 g silica sol particles and the resulting mixture was ultrasonically irradiated for 20 min to form uniform solution. The obtained uniform solution was transferred to a Teflon- line stainless steel autoclave, which was sealed and maintained at 190°C for 24 h, and then air-cooled to room temperature. The resulting yellow precipitate was collected, washed with distilled water, and dried in air at room temperature. The yellow precipitate was treated with 0.5 M NaOH solution to remove the silica sol.
3.3. Schematic Procedure Used to Synthesize CeO2 Hollow Microspheres
- Scheme 1 shows the overall procedure used to synthesize CeO2 hollow microspheres. Before hydrothermal treating of the reaction solution, silica microspheres are dispersed in Ce(NO3)3 solution by ultrasonic vibration. Under hydrothermal conditions, Ce(NO3)3 were decomposed to form CeO2 nanocrystals on the surfaces of silica microspheres. The basic reaction may be expressed as follows:
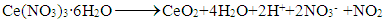
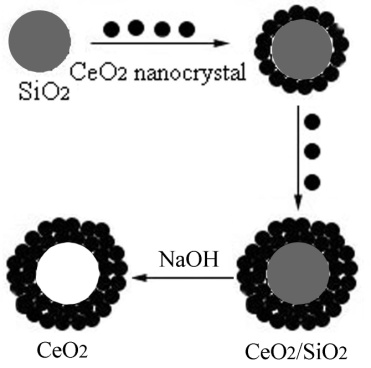 | Scheme 1. Schematic procedure used to synthesize CeO2 hollow microspheres |
4. Result and Discussion
4.1. X-ray Powder Diffraction (XRD) Analysis
- X-ray powder diffraction (XRD) analysis was carried out to investigate the phases of the as–synthesized products. A typical XRD pattern of the as-synthesized products is shown in Figure 1. All of the diffraction peaks in Fig. 2 can be exactly indexed to the face-centered CeO2 with lattice constants a = 5.410 Å, which are in good agreement with the literature values (JCPDS 34-0394). [14]
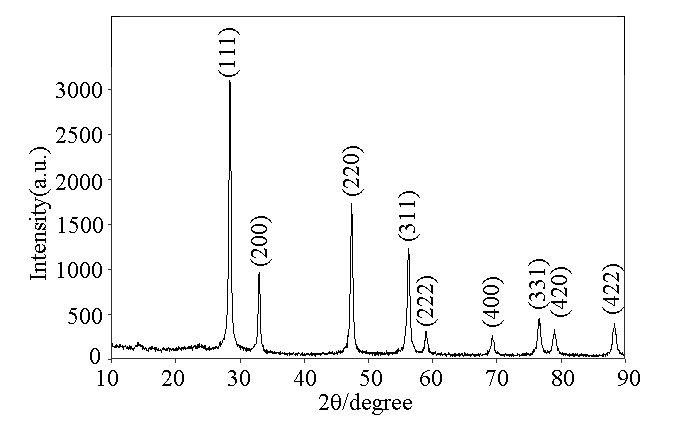 | Figure 2. XRD patterns of as-synthesized CeO2 hollow spheres |
4.2. SEM Analysis of as-synthesized CeO2 Hollow Spheres
 | Figure 3. SEM image of as-synthesized CeO2 hollow spheres synthesized at the concentrations of 0.1M Ce(NO3)3 |
4.3. TEM Analysis of as-synthesized CeO2 Hollow Spheres
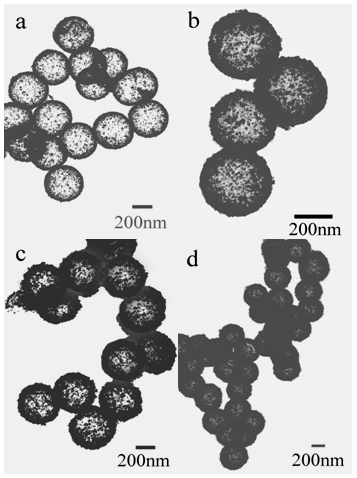 | Figure 4. TEM images of as-synthesized CeO2 hollow microspheres: the thickness of shell is (a) 20 nm (b) 35 nm, (c) 45 nm, and (d) 50 nm, respectively |
|
4.4. UV/vis Analysis of as-synthesized CeO2 Hollow Spheres
- As ultraviolet blocking materials, CeO2 has strong absorption properties in the ultraviolet range. Fig. 5 shows the UV/vis absorption spectra of the CeO2 powders. As the shell thickness increases from 20 nm to 50 nm, the absorbing boundary of CeO2 hollow microspheres is blue-shifted from 450 nm to 430 nm. The strong absorption in the spectra is due to the charge-transfer transition from O2- (2p) to Ce4+ (4f) orbitals in CeO2, which overruns the well-known f to f spin-orbit splitting of the Ce 4f state. [15-17] To the best of our knowledge, there was no report on them. The optical properties of CeO2 hollow microspheres will prevent some damage from ultraviolet rays. Compared with some previous work on ultrafine CeO2 nanoparticles, their strong absorption band is below 400 nm in the spectrum. [18] As a result, the high absorption in the UV region for CeO2 hollow microspheres indicates that this material was suitable as a UV-blocking material.
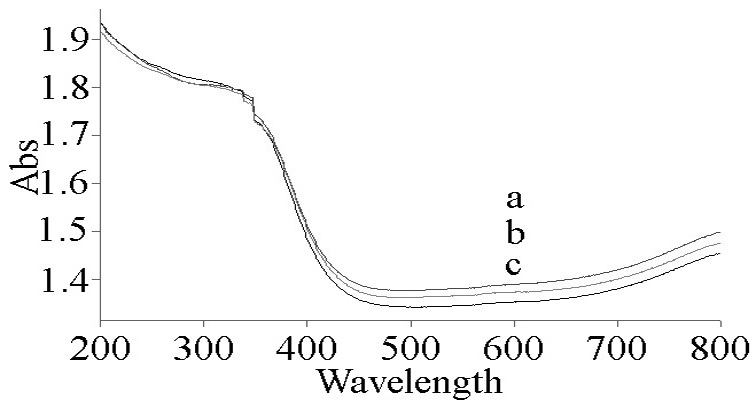 | Figure 5. UV–vis absorbance spectra of CeO2 hollow microsphere powder: the thickness of shell is (a) 20 nm, (b) 45 nm and (c) 50 nm |
ACKNOWLEDGEMENTS
- This work was financially supported by High quality curriculum project of graduate education in Shandong China (SDYKC17036), Upgraded Project of Shandong Province for Guidance Ability of Graduate Tutors (SDYY16014, SDYY15035), and National Training Programs of Innovation and Entrepreneurship for Undergraduates, China (201710426061).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML